3 Nov 2020
David Rendle and Richard Newton use the 2019 virus outbreak to discuss the challenges of controlling future instances and the need for greater prevention.

The rapid spread of equine flu (EI) across the UK in 2019 highlighted the potential disruption and economic impact of the disease.
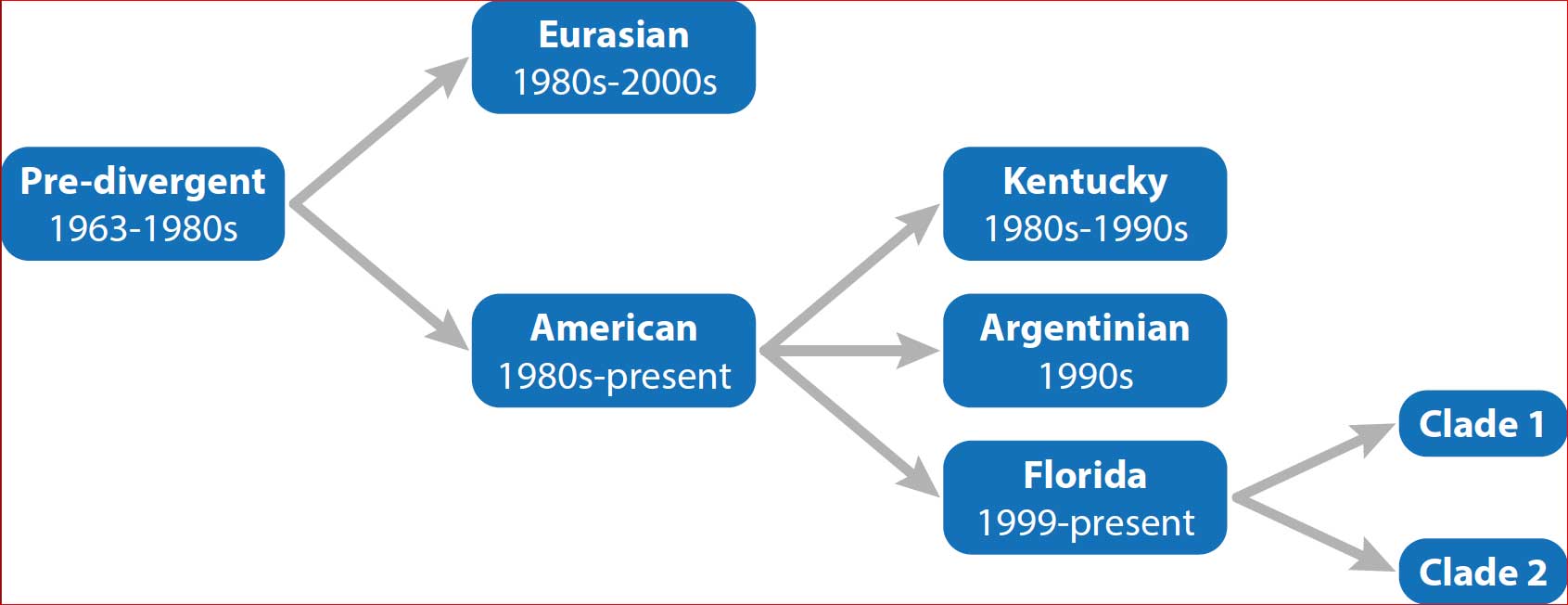
The UK, like many northern European countries, saw Florida sublineage clade 1 (FC1) H3N8 EI virus transmit within its equine population – resulting in morbidity and occasional mortalities across at least 234 UK equine premises, on which 412 animals were confirmed by laboratory testing with the infection1.
Although 72% of the infected horses were unvaccinated and demonstrated clinical signs, 18% of horses that tested positive had been appropriately vaccinated with one of the three UK registered EI vaccines; the remaining 10% were either of lapsed or unknown vaccination status (Whitlock and Newton, unpublished data).
The events of 2019 again highlighted the value of vaccination in limiting the spread and impact of EI. These experiences supported the results of mathematical modelling of the disease performed previously by the AHT and University of Cambridge, which demonstrated that in vaccinated populations more than 80% of outbreaks resolve with fewer than 5% of horses being infected2. Modelling the disease also demonstrated that six-monthly vaccination significantly reduced the risk of an outbreak on a training yard following the admission of an infected horse2.

An international epidemic due to an FC1 EI virus had not been predicted, given that:
The two market-leading UK EI vaccines both contained representative FC1 strains, but only one included an FC2 strain (Table 1). Since 2010 the World Organisation for Animal Health (OIE) has – through an expert surveillance panel that reviews EI virus vaccine strain selection – recommended an FC2 strain be included in vaccines in addition to an FC1 representative virus3, which was first recommended in 2004.
| Table 1. Equine influenza vaccines registered for use in the UK | ||||
|---|---|---|---|---|
| Vaccine | Manufacturer | Nature | Adjuvant | Composition |
| Equilis Prequenza | MSD Animal Health | Whole inactivated | ISCOM-matrix | Clade 1 (South Africa/4/03) Eurasian (Newmarket/2/93) |
| Equip F | Zoetis | Subunit | ISCOM | N7N7 Eurasian (Borlange/91) American (Kentucky/98) |
| ProteqFlu | Boehringer Ingelheim | Recombinant canarypox | Carbomer | Clade 1 (Ohio/03) Clade 2 (Richmond/07) |
| ISCOM = immune-stimulating complex | ||||
Globally, FC1 viruses have been dominant more recently and the relative importance of FC2 virus appears to be diminishing. In the 12 months to April 2020 only one FC2 outbreak was reported to the expert surveillance panel and this occurred in China1.
Although it would be far too early to suggest that inclusion of FC2 virus in vaccines is no longer necessary, it is known for lineages of EI viruses to become presumptively extinct when overtaken by more dominant strains and occasionally be dropped from vaccines.
FC1 now appears to be the predominant EI threat globally and, given the extent of the European epidemic, the virus that circulated in 2019 appeared to be more pathogenic than the FC2 viruses that had been circulating over the previous 20 years.
Haemagglutinin (HA) gene sequencing of the FC1 H3N8 virus identified in the early French EI cases detected at the end of 2018 – and subsequent cases in France, the UK and Ireland – revealed two amino acid substitutions from the FC1 strains isolated in South America in 2018 and more than 10 amino acid substitutions from the closest EI vaccine strains, with several of the substitutions being located in antigenic sites4.
Unfortunately, detailing genetic sequence differences does not provide a reliable indication of the threat that naturally evolving EI viruses pose to the vaccinated equine population5; this can only be established by performing vaccination-challenge studies.
Antigenic changes in the HA glycoprotein on the surface of EI viruses are primarily determined by haemagglutination inhibition (HI) assays with ferret – rather than equine – antisera because ferrets are known to mount more strain-specific responses than horses, which better predict vaccine and infecting strain mismatches5,6.
Although the latest FC1 viruses have gradually diverged genetically from the OIE‑recommended vaccine strains, viral HI data using post-infection ferret sera has indicated they continue to remain antigenically similar to the viruses recommended for inclusion in the vaccines.
Therefore, in April 2020 the OIE expert surveillance panel agreed no scientific justification existed – based on established criteria – for revising the recommendations on vaccine composition, although it also agreed to keep this under close review in the interim before its next scheduled annual meeting in spring 20211.
It has long been known that virus strain composition is only one determinant of EI vaccine efficacy.
If all EI vaccines were created equal in terms of technology, it would be logical to select the vaccine with the most current virus strain composition. However, important differences exist in the technology used in UK‑registered EI vaccines (Table 1).
ProteqFlu contains live‑attenuated canarypox expressing HA, but no other EI viral proteins; Equip F is a subunit vaccine containing multiple EI viral proteins with an immune‑stimulating complex (ISCOM); and Equilis Prequenza contains whole inactivated virus within an ISCOM‑matrix. All three vaccines have been shown to stimulate cell-mediated immunity and mucosal defences in addition to stimulating a humoral response.
The degree to which different technologies stimulate cell‑mediated immunity and mucosal defences is debated in both equine and human medicine. Humans do not well tolerate the inclusion of adjuvants; therefore, human vaccines rely greater on maintaining influenza strains that are very closely related to field strains.
Furthermore, equine influenza is considered to evolve relatively slowly, particularly when compared to human influenza7.
The breadth of the immune response generated by the vaccine is dependent on the nature and number of antigens within the vaccine. Therefore, a vaccine with a limited number of antigens could see its efficacy reduced if genetic mutation is present that results in a change in the target proteins.
ProteqFlu is entirely dependent on an immune response to two HA proteins that have been encoded within separate canarypox vectors, while Equip F and Equilis Prequenza contain multiple viral antigens as subunits and whole inactivated viruses, respectively.
The technical challenges associated with updating ISCOM or ISCOM-matrix vaccines is greater and, as a result, more costly; in vectored vaccines the HA can be relatively readily substituted, while with ISCOM or ISCOM‑matrix vaccines all the subunits or whole virus have to be changed.
However, a limit exists to the number of genes that can be encoded within the pox vector – and as those encoding HA are known to mutate naturally at a relatively high rate, a greater need therefore exists to ensure the HA sequence in ProteqFlu is closely aligned with field strains by regularly updating the vaccine.
A recent study demonstrated that an ISCOM-matrix vaccine containing European and FC1 virus strains (Equilis Prequenza) provided a similar level of protection to a canarypox‑vectored vaccine (ProteqFlu) that contained the HA genes of both FC1 and FC2 viruses, against infectious viral challenge with a recently circulating FC2 strain8.
The MSD Animal Health-funded study, which was performed in the UK under Home Office licence, evaluated immunity at approximately four months (120 days) after administration of the second dose of the primary vaccination course; towards, but not quite in, the classic “immunity gap” when immunological responses to vaccines are at their weakest – just prior to the earliest possible revaccination (at 150 days) currently allowed under most equestrian regulations and when the risk of infection is highest.
Historically, vaccination-challenge studies have been performed only a few weeks after the second vaccination of the primary course when vaccine‑stimulated immune responses are known to be optimal.
The two vaccines induced similar antibody titre profiles as assessed by single radial haemolysis, HI and virus neutralisation to both FC1 and FC2 strains. However, as previously highlighted, based on these serological methods horses produce readily “cross‑reacting” antibodies against quite markedly antigenically different viral strains, but these do not equate to equivalent cross‑protection to viral challenge9.
Consequently, extreme caution is recommended when interpreting any claims of likely equivalent “performance” between vaccines with different strains based solely on serology.
Commendably, however, the study also assessed the performance of both vaccines to viral challenge in preventing clinical signs of EI and reducing viral shedding (and, therefore, risk of disease transmission). Furthermore, the challenge infections were performed several months after the expected peak of immunity.
In all, 19 Norwegian Fjord horses received a challenge dose with FC2 virus (Wexford/14) provided by the Irish Equine Centre; seven horses had been vaccinated with Equilis Prequenza, seven with ProteqFlu and five remained unvaccinated. Investigators were blinded to the treatment group allocations and performed daily clinical assessments, grading seven different clinical signs – including rectal temperature, cough and nasal discharge – and nasal swabs were collected to quantify virus shedding.
Not unexpectedly, even with the small group sizes, both vaccines demonstrated highly significant reductions in clinical signs and viral shedding compared to the unvaccinated controls. However, some encouraging findings also arose from the limited number of challenged vaccinated animals that suggested at least equivalent performance by the non-FC2 whole inactivated virus ISCOM-matrix adjuvanted Prequenza with the FC1/FC2 HA only-based ProteqFlu in an FC2 EI virus challenge.
These findings perhaps, therefore, help explain why no notable evidence had arose in the preceding decade – when FC2 EI virus strains were dominant in the UK – of failure of EI vaccine efficacy among horses last vaccinated with either ProteqFlu or Prequenza, even though no FC2 strain is represented in Prequenza.
The study8 also addressed an important observation by Daly et al (2007)10 that no published reports existed of experimental evaluation of the ability of commercially available vaccines to protect against challenge with a variant strain at a later time point after peak response to primary vaccination.
While this new study8 may be seen as commercially motivated, it raises some fundamental questions over how we view the relative importance of virus strain updates in EI vaccines; not least as these updates take time and large investment by their manufacturers.
It would undoubtedly be desirable for all EI vaccines to always contain virus strains that are fully representative of those strains horses are likely to face in the field; however, the reality is that annual updates – as occurs with human flu vaccines – are not practically feasible within the constraints of the relatively limited EI vaccine market and neither are they scientifically necessary.
As we head to the end of 2020 – a year dominated by one particular virus and the clamour for vaccines to control it – the irony is that FC2 EI virus, on which this study was focused, has not actually been identified in Europe for almost three years. After an exceptionally quiet year for EI in 2018, Europe and the UK in particular saw the incursion and wide spread of an FC1 virus in 2019 – a strain that was apparently adequately represented in two market-leading vaccines.
All of this confirms the difficulties faced in accurately predicting and controlling biological events such as epidemics and pandemics, and that from each of them new perspectives and lessons are inevitably learned that may improve future disease control effectiveness.
In concluding, while we may debate the relative importance and timeliness of EI virus vaccine strain updates, the events of 2019 again affirm that subtle differences in efficacy between vaccines are largely inconsequential when factored against the dismal rate of vaccine uptake in the UK, which currently stands, at best, at about 40%.
The UK – with its high number of horse movements and variable compliance with implementation of effective biosecurity and disease prevention protocols, resulting in a suboptimal level of national herd vaccination coverage to EI – undoubtedly remains at risk of future incursions of EI, irrespective of the make‑up of our EI vaccines.