14 Dec 2020
Abdominocentesis in horses
Veronica Roberts discusses indications, techniques, risks and sample interpretation around this common equine procedure.
Abdominocentesis is a procedure commonly performed in equine practice. This article covers the main indications for performing abdominocentesis.
It will describe the two techniques and then cover the risks, which will help enable you to determine which technique is most suitable for you and for the individual case.
Finally, basic guidance will be given on interpretation of sample results.
Indications
The main indication for abdominocentesis is in the investigation of abdominal disease, as analysis of peritoneal fluid reflects the changes that occur within the abdomen.
The technique can be used in the determination of the need to perform surgery in acute abdominal pain, as well as part of the investigation for weight loss and inflammation of unknown origin. It is used in the diagnosis of peritonitis, haemoabdomen and some forms of abdominal neoplasia.
As with any procedure, before performing it, consider whether your intended actions with the case will be influenced by the results, and whether the risks outweigh the benefits.
For colic cases, this would mean performing the procedure only if other tests, including physical and rectal examination, have been unable to provide a clear decision on whether emergency exploratory laparotomy is indicated.
The procedure should not be performed in cases with severely distended intestines due to the increased risk of enterocentesis.
Preparation
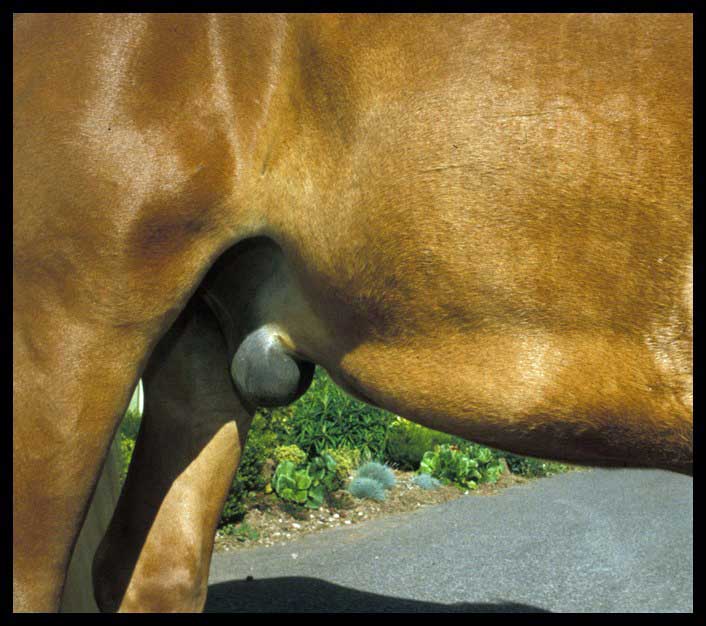
Choose your site to be at the most dependent part of midline. Ideally, the site is 2cm to 3cm to the right of midline (to help avoid the spleen), but in a horse with a moderate to large amount of abdominal fat, you may need to go through the linea alba to be able to get deep enough to access peritoneal fluid.
Try to avoid areas with obvious superficial veins. If available, abdominal ultrasound can be used to help identify a suitable site, with a pocket of free fluid and away from intestine.
The horse should be under control, and the author would usually sedate and sometimes apply a nose twitch. To reduce the chances of getting kicked, stand at the horse’s shoulder. For a right-handed operator, stand at the right shoulder.
Stand parallel to the horse facing the tail, but then turn 45° to the left, so you are angled away from the hindlegs. To crouch, stretch your left leg forwards and right back, so that the leg closest to the horse is out of line with a forward kick from the hindlegs.
The site should be clipped and aseptically prepared. Sterile gloves should be used for the procedure (Panel 1).
- So as not to surprise the horse, the author makes sure she palpates and presses the area where she is going to insert the needle for sampling or for the local anaesthetic bleb, just before doing so.
- Have the lids off the tubes ready to catch fluid, before you start.
- Collect peritoneal fluid into a plain tube first and then an ethylenediaminetetraacetic acid (EDTA) tube. This way, if fluid stops dripping after only one tube has been collected, you can still decant some of the fluid from the plain tube to the EDTA for cytology; you cannot do the same the other way around.
- You don’t always get fluid, even with a teat cannula.
Technique
Abdominocentesis can be performed with either a needle or a teat cannula.
Needle technique
The author usually uses a 0.5mm (21G), 5cm (2in) needle. In an obese horse, you may need to use a spinal needle to access fluid, but she would not advise doing this without having performed abdominal ultrasonography first to choose your site and reduce risks.
After you have identified and prepared the sampling site, insert the needle in a vertical direction. Start by inserting the first 1cm to 2cm quite quickly. In case the horse reacts, be prepared to rapidly let go of the needle and withdraw your hand. Usually once the needle is through the skin they no longer react and you can then maintain contact with the hub of the needle.
Advance the needle 0.5cm at a time, still in a vertical direction, either until fluid starts to drip from the hub or until the needle is inserted all the way up to the hub (Figure 1). If no fluid is present, try to rotate the hub of the needle. If still no fluid is present, gently redirect the needle. If still no fluid is present then usually the author withdraws the needle and tries again in a slightly different place.

Aspiration with a syringe seems logical, but is rarely rewarding – probably due to occlusion by omentum or peritoneum. If still no fluid is present, consider using a teat cannula.
Fluid should usually drip out easily. If only a very slow drip occurs or fluid just sits in the hub, then this may be gastrointestinal content. Withdraw the needle immediately and confirm your suspicions by observation of the fluid.
Cannula technique
Insert a 0.2mm (25G), 1.5cm (5/8in) needle under the skin at the selected sampling site and inject in a bleb of local anaesthetic. The author uses 2ml of two per cent mepivacaine.
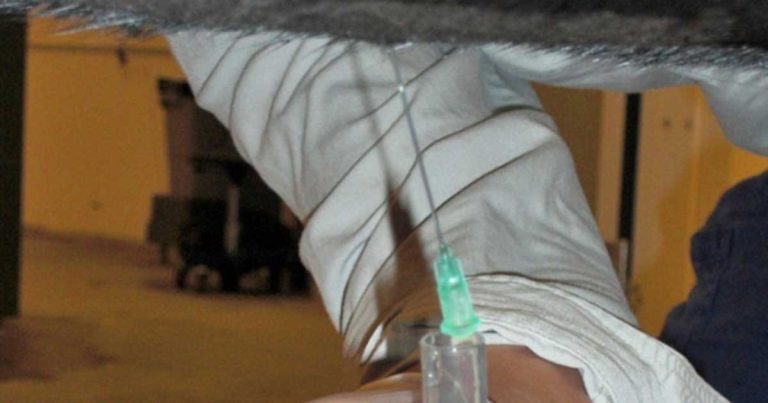
Insert the cannula through a sterile swab (Figure 2) to reduce contamination from cutaneous bleeding into the sample pot. Then, using a guarded 15 scalpel blade, make a small stab incision through the skin, to a depth of about 1cm into the body wall.

The teat cannula should be pushed gently through the incision and then pushed through the body wall with a short, sharp thrust.
Risks
With any procedure, a risk:benefit analysis is part of the decision-making process on whether to perform the procedure. Overall, it is a low-risk procedure.
The balance of risks with the two techniques is slightly different, which can influence which technique you choose.
It is the author’s interpretation that one technique is not overall a greater risk than the other, so choice can often be based on personal preference and practicality; for example, the needle technique is simpler to perform and so may be more suitable than the cannula technique when in the field (Table 1).
| Table 1. Complications and appropriate actions | |||
|---|---|---|---|
| Complication | What can happen? | What to do? | How to avoid it happening? |
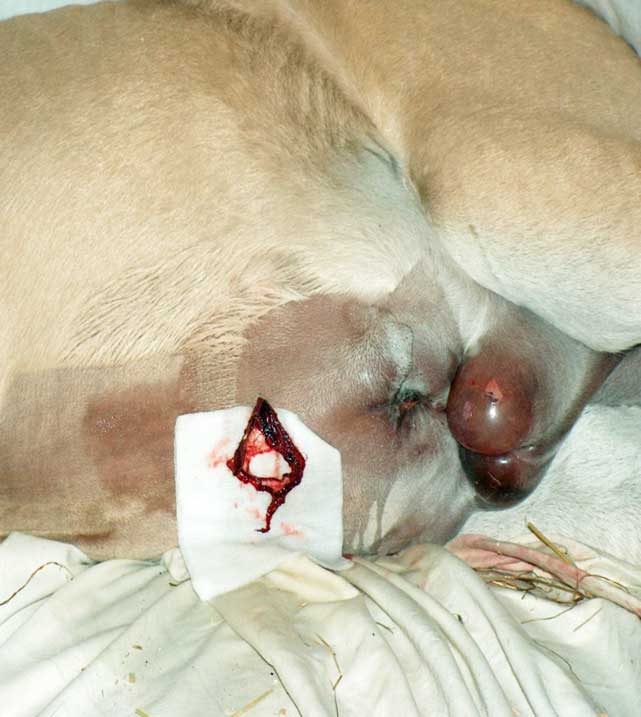
| Blood in sample (Figure 3) | Blood in the sample may be due to perforation of the spleen, haemoabdomen, blood contamination from a superficial vessel or sanguineous exudate from compromised intestine. | To tell if it is splenic blood, compare the PCV to that of peripheral blood. Blood from the spleen has a higher PCV. If it is from the spleen, insert a new needle at a different site to obtain a peritoneal sample. | Place the needle just to the right of the midline. A longer needle may be required in horses with a thick layer of subperitoneal fat. |
|
|
|||
| Cellulitis (Figure 4) | Infection and inflammation of the SC tissues. Severe cellulitis reported in 2/850 samples (Tulleners, 1983). | Treat with broad-spectrum antibiotics – for example, IV penicillin or gentamicin, or oral trimethoprim-sulfadiazine. Phenylbutazone or flunixin may be given for analgesia. | Ensure a sterile technique is performed. |
|
|
|||
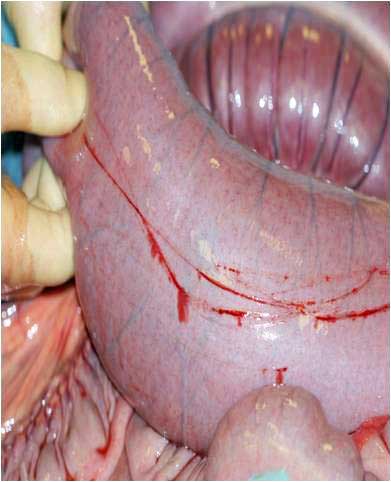
| Enterocentesis (needle; Figure 5) | This can occur in two per cent to five per cent of cases performed with a needle (Tulleners, 1983). Figure 5 shows superficial scratching of the intestinal wall, but if deeper, this could be a laceration. The horse moved abruptly during sampling. | Usually this is an insignificant sequelae. Clinical peritonitis may develop in rare cases. A localised peritonitis is likely to occur and previous recent enterocentesis should be considered when interpreting future samples (Schumacher et al, 1985). | Ensure suitable restraint of the patient. If intestinal contents (dark brown, turbid and malodorous) appear in the needle hub, withdraw the needle. |
|
|
|||
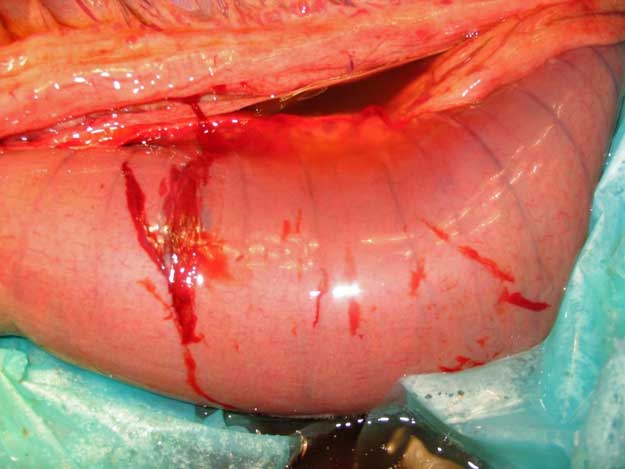
| Enterocentesis (cannula; Figure 6) | This is a rare, but much more serious penetration of the intestines with a cannula. Healthy gut will heal quickly, but not if compromised. | Antibiotic therapy as for peritonitis – or even an exploratory laparotomy – may be required in severe cases. | Hold the needle loosely; do not walk or “shake” the horse. Consider whether to still tap if feel distended viscus on rectal exam. |
|
|
|||
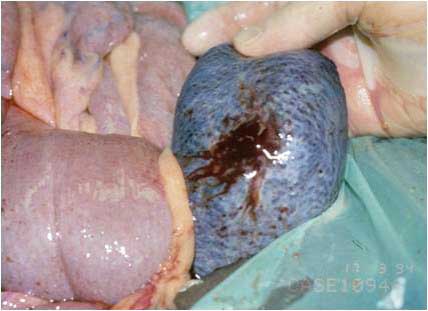
| Extrusion of omentum (Figure 7) | If too large a stab incision is made with the scalpel blade the omentum may extrude through the hole. | Ligate, clean, replace, suture. May need exploratory laparotomy if very severe. | Care with the size of stab incision for the cannula tip. |
|
|
|||
Interpretation
Start with visual inspection of the fluid. In cases of colic, peritoneal lactate is a sensitive indicator, changing sooner than peripheral lactate, for the detection of ischaemic intestine (Latson et al, 2005).
Lactate can be measured horse-side with a simple lactate meter. Total protein can be measured horse-side with a refractometer. Evan a basic in-house cytology following Diff-Quik staining can be helpful for major changes where a rapid result is required. Further information can be gained by a specialist clinical pathologist.
| Table 2. Interpretation of abdominal fluid cytology | ||||
|---|---|---|---|---|
| Gross appearance | Total protein (g/l) | Nucleated cell count (×109/L) | Cytology | |
| Normal | Clear-yellow, odourless | <2.5 | <5 | 2:1 ration of non-degenerate neutrophils to macrophages, no red blood cells (RBCs). |
| Simple obstruction | Odourless, clear-turbid, pale yellow | 2.5-3 | 3-8 | Predominantly non-degenerative neutrophils. |
| Strangulating obstruction | Serosanguineous, turbid | 3.5-6 | 3-8 | Degenerate neutrophils +/− intracellular or extracellular bacteria, RBCs. |
| Intestinal rupture | Malodorous, turbid, green to brown | 3.5-6 | <2 | Degenerate neutrophils +/− intracellular or extracellular bacteria, plant material, protozoa, RBCs. |
| Peritonitis | Thick turbid, dark yellow to orange | >2.5 | >10 | High numbers of degenerate and non-degenerate neutrophils, intracellular and extra-cellular bacteria. |
Conclusions
Abdominocentesis is a low-risk, but not risk-free, procedure that can add valuable information to the investigation of some cases in equine practice. It is a relatively simple procedure that can be performed – and, to a degree, interpreted – in the field.
References
- Latson KM, Nieto JE, Beldomenico PM and Snyder JR (2005). Evaluation of peritoneal fluid lactate as a marker of intestinal ischaemia in equine colic, Equine Vet J 37(4): 342-346.
- Schumacher J, Spano JS and Moll HD (1985). Effects of enterocentesis on peritoneal fluid constituents in the horse, J Am Vet Med Assoc 186(12): 1,301-1,303.
- Stephen J (2008). Abdominocentesis. In Corley K and Stephen J (eds), The Equine Hospital Manual, Blackwell Publishing, Oxford: 16-17.
- Tulleners EP (1983). Complications of abdominocentesis in the horse, J Am Vet Med Assoc 182(3): 232-234.