28 Mar 2022
Best practice for worming and anthelmintic resistance
David Rendle outlines how veterinary professionals, and clients, can treat and reduce these common parasitic infections in horses.
Questionnaire surveys of horse owners have indicated that most value their vet’s opinion on de-worming, but few actually use them as their primary source of advice (Stratford et al, 2014).
Although an increasing number of owners are aware of the need to target anthelmintic treatments to reduce the development of resistance and ecotoxic effects, a minority seem to put these principles into practice appropriately.
Under the current system of prescribing, there will always be challenges engaging with owners who find it easier to shop online or at their local equestrian retailer (Furtado and Rendle, 2021); however, ample scope exists for veterinary practices to be more engaged in planning de-worming strategies and preventive medicine policies in general.
It is the view of BEVA that all horse owners should have a veterinary-led parasite control programme for their horse(s), and horse owners should only use de-wormers where they are essential to reduce the risk of clinical disease.
Veterinary surgeons are uniquely equipped to formulate herd plans that take an holistic view of the management on the property, factoring in:
- Population dynamics.
- Horse movements.
- Pasture management.
- Stocking density.
- Recent de-worming history.
- Episodes of parasite-related disease.
- Local resistance patterns.
- Results of diagnostic testing.
- Best evidence for using anthelmintics, whether this is in accordance with datasheet recommendations or not.
Improving management practices
Simple measures can be implemented to reduce the need for anthelmintics on equestrian properties:
- Minimise stocking density.
- Maintain consistent horse populations.
- Use faecal egg counts (FEC) to identify animals that persistently shed.
- Collect faeces at least twice-weekly.
- Rest and rotate pasture (particularly on stud farms).
- Avoid using the same pastures for young stock repeatedly.
- Prevent the development of roughs where horses repeatedly defecate.
- Keep dung heaps away from grazing areas.
- Co-graze with ruminants.
- Treat new horses on arrival with moxidectin and praziquantel before quarantining them for three to four days, and dispose of faeces rather than spreading it on the property. Hopefully this forms part of a longer period of quarantine (two to three weeks) aimed at preventing the introduction of bacterial and viral infections.
Sustainable control of cyathostomins through the grazing season
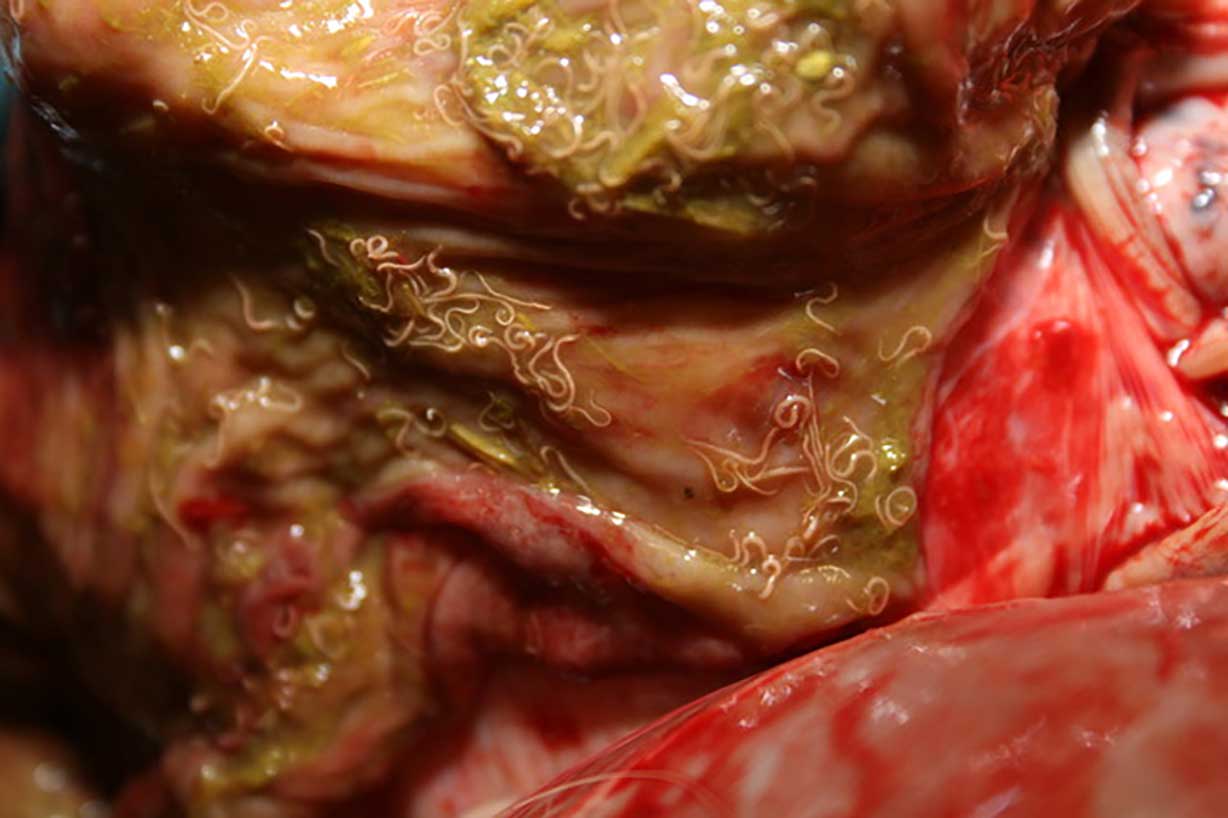
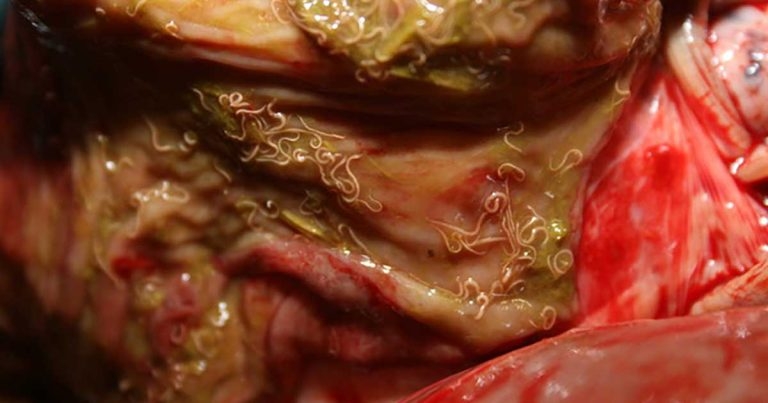
Cyathostomins are the most important cause of parasitic disease in adult horses and their control is, therefore, the focus of any equine de-worming strategy in a mature horse population. Individual FEC should be the central pillar of parasite control through the grazing season, and they should inform the need for treatment in individual horses (Figure 1).

It would be helpful if consistency is evident of the message around the required frequency of worm egg counting and appropriate “cut-offs”.
The evidence is not clear-cut, and these parameters are also influenced by the nature of the population and the risk of infection.
As a rule of thumb, FEC should be used every two to three months between March and September with horses being treated if their counts are above 250 to 500 (Rendle et al, 2019). The lower the risk in the population, the higher the treatment threshold that can be used.
Over winter, egg shedding decreases, horses generally spend more time stabled and many will have received a “larvicidal” anthelmintic with a long egg reappearance period, so less value exists in performing FEC during this time.
When performing FEC, it is important that faeces is handled appropriately by following this procedure (Rendle et al, 2019):
- Collect samples for individual horses within 12 hours of excretion.
- Take one to three samples from at least three different balls of faeces to generate a sample the size of a table tennis ball (40g to 50g).
- Place the sample in a zip-lock bag and expel the air prior to sealing.
- Keep the sample refrigerated prior to posting.
- Ensure the sample is analysed within five days of collection, preferably within two days.
- Ensure the sample is mixed thoroughly prior to processing.
Faecal egg count reduction tests (FECRT) should be performed annually on every property to investigate and monitor resistance patterns. Cyathostomin resistance to benzimidazoles is widespread (if not ubiquitous) in the UK and this drug class should not be relied upon to control cyathostomin infections unless FECRT have been performed to demonstrate efficacy.
Although pyrantel resistance is wide-spread, it appears to be efficacious against some populations of cyathostomins and should be used where the results of FECRT confirm efficacy.
Cases of ivermectin and moxidectin resistance are now being reported with egg reappearance periods becoming shorter. Annual rotation of FECRT should be performed with a different anthelmintic being tested each year.
FECRT necessitates an FEC being performed prior to, and 10 to 14 days after, anthelmintic treatment of those horses with a high FEC. If reduction in egg count is insufficient (Table 1), then treatment failure (such as failed administration or under-dosing), or resistance are suspected and should be further investigated with repeat FECRT.
| Table 1. Expected strongyle faecal egg count reduction and egg reappearance periods following treatment in sensitive worm populations according to product datasheets | ||
|---|---|---|
| Drug | Expected faecal egg count reduction | Expected egg reappearance period |
| Fenbendazole | More than 90% | Six to eight weeks |
| Pyrantel | More than 90% | Four to six weeks |
| Ivermectin | More than 95% | Six to eight weeks |
| Moxidectin | More than 95% | Twelve weeks |
Resistance cannot be determined reliably unless results are available for at least 6, preferably 10, horses. A good formula to calculate group FECR as a percentage is:
Pre-treatment group mean FEC – Post-treatment group mean FEC × 100
Pre-treatment mean FEC
Ivermectin and pyrantel are currently the preferred options for routine treatment in response to a high FEC result.
Pyrantel has the potential advantage that it reduces selection pressure against macrocyclic lactones, has efficacy against ascarids and will have an effect on Anoplocephala perfoliata even at a routine (single) dose (Lyons et al, 1989), which may reduce the overall need for anthelmintics.
Ivermectin has the advantage that it will kill other parasite species such as large strongyle larvae, as well some cyathostomin larval stages and Gasterophilus species.
Traditionally, it was advised to move treated horses to fresh pasture (“dose and move”) to reduce numbers of helminths transmitted to clean grazing. This serves to reduce refugia, thereby increasing selection pressure and is contraindicated on most properties with reasonable management. On poorly managed properties where levels of infection are high, dose and move strategies may still be needed to reduce the number of eggs shed on to the new pasture, and thereby reduce total anthelmintic use after horses have moved.
Medium to long-term, it is important that management practices are improved to enable refugia to be maintained and to reduce the drive toward resistance.
Autumn/winter treatment of encysted cyathostomins
Traditionally, blanket treatments for cyathostomin larvae have been used in the autumn and/or winter with additional consideration being given to the elimination of tapeworms (such as Anoplocephala species).
Blanket treatment of all horses in the autumn and winter can no longer be justified, and the need for treatment should be considered on the basis of risk (Table 2) and recent diagnostic testing results.
Routine treatment should be spared in populations that are low-risk and have consistently low FEC. Serological tests have been developed to assess cyathostomin burden, and hence the need for larvicidal dosing; however, there is insufficient validation data for the test and experience from the field suggests that literal interpretation of the test results leads to over-treatment.
This undermines efforts to reduce anthelmintic use and, therefore, many clinicians rely on assessment of risk (Table 2) and the population’s recent worm egg count results rather than results of cyathostomin antibody levels.
| Table 2. Assessing horses as low, medium or high risk of parasitic disease can help in determining the need for diagnostic testing and treatment | ||
|---|---|---|
| Low risk | Moderate risk | High risk |
| Repeated negative faecal egg count (FEC) | Low-to-moderate FEC | High FEC |
| Low antibody levels | Moderate antibody levels | High antibody levels |
| Negative FEC and antibody levels in cohorts | Negative FEC and antibody levels in cohorts | Negative FEC and antibody levels in cohorts |
| Aged 6 years old to 15 years old | Aged more than 15 years old | Aged less than six years old |
| Faecal collection more than or equal to twice per week | Sporadic faecal collection | No faecal collection |
| Good pasture management | Moderate pasture management | Poor pasture management |
| Stable population | Occasional movement | Transient population |
| Low stocking density | Medium stocking density | Medium stocking density |
| No youngstock | Grazing with youngstock | Grazing with youngstock |
| Effective quarantine and treatment on entering | No quarantine and treatment on entering | No quarantine and treatment on entering |
| No history of parasitic disease | History of parasitic disease | History of parasitic disease |
| No history of colic | History of colic | History of colic |
| No evidence of anthelmintic resistance | Resistance to anthelmintics | Anthelmintic resistance identified on site by faecal egg count reduction test |
Adult horses (more than six years old) develop robust (but variable) immunity to cyathostomins and we frequently forget they can tolerate relatively large burdens of cyathostomins without developing clinical disease (Nielsen 2012).
Furthermore, no evidence exists that the administration of larvicidal anthelmintics in the autumn or winter reduces the risk of clinical disease associated with mass emergence of cyathostomins in the winter or spring.
The best means of reducing the risk of larvicidal disease is to ensure good management and limited exposure to cyathostomins through the grazing season (Figure 2).
Less than 50% of adult horses in the UK are infected with adult tapeworms (Morgan et al, 2005; Pittaway et al, 2014; Lightbody et al, 2016) and in the majority, infection is sub-clinical. Colic associated with tapeworms is relatively uncommon in adult horses and the risk of tapeworm-associated disease in adult horses is not well quantified (Nielsen, 2015).
In younger horses, particularly on stud farms, tapeworm-associated disease is more common and annual or bi-annual monitoring is indicated. Treatment should only be administered to adult horses in response to positive serum or salivary antibody testing or evidence of clinical infection.
Resistance among tapeworms is suspected, but has not been demonstrated conclusively, given the difficulties in identifying infection.
Praziquantel is the preferred treatment for tapeworms to reduce unnecessary exposure of strongyles to pyrantel. Praziquantel paste is no longer available as a registered product, but is available as a “special” formulation.
Horses that have received a single dose of pyrantel within recent months may not need further treatment for tapeworms.
Other parasites
Gasterophilus species are not considered a cause of disease unless they are present in extreme numbers. They do not require routine treatment. Where infection is identified, fly control should be improved.
Oxyuris equi appears to be an increasingly common cause of perineal pruritus. It is not known whether this is due to resistance, climate change or reduced anthelmintic use associated with targeted worming programmes.
Knowledge on efficacy of different classes of anthelmintic against O equi is limited and it is not possible to recommend a specific treatment. Hygiene and decontamination of the environment are paramount. The perineal region should be cleaned once, preferably twice-daily to remove eggs and break the life cycle.
Application of petroleum jelly to the perineum may prevent eggs from sticking and hinder parasite migration. The indiscriminate and/or frequent use of different classes of anthelmintics to eliminate O equi is strongly discouraged. No indication to apply anthelmintics to the perineum or to insert them into the rectum exists.
Strongylus vulgaris has increased in prevalence in association with reduced use of anthelmintics in mainland Europe. This “re-emergence” has not been identified as a cause of disease in the UK, but remains a potential concern. Annual ivermectin or moxidectin administration is sufficient to eliminate larval stages of large strongyles. If pasture is well managed, FEC are performed and exposure to other parasites is repeatedly low, then the risk of verminous arteritis is low.
Clinical disease associated with Fasciola hepatica is rare in horses, even when they are grazed with ruminants that are infected.
Sub-clinical liver disease is common in horses and other causes of hepatopathy are far more likely to be responsible for increases in liver enzymes. Exposure to fluke can be determined using a recently developed serological assay from the University of Liverpool; however, no means of confirming clinical infection in horses exists.
Dictyocaulus arnfieldi (lungworm) should not be considered relevant to horses in the UK, unless donkeys are present on the same pasture, in which case the risk of infection should be factored into the parasite control programme. Dictyocaulus arnfieldi is highly sensitive to ivermectin.
Youngstock
Immunity to parasites increases up to the age of five or six years and then wanes in horses in their late teens (Figure 3). The majority of clinical larval cyathostominosis cases occur in horses that are one years to three years of age (Love et al, 1999). Clinical disease associated with ascarid infection is also being reported in older animals, where once it was considered a disease of foals. Where youngstock are on a property, consideration should be given to:
- Increasing the frequency of FEC.
- Being more proactive in collecting faeces and rotating grazing.
- Routine treatment for cyathostomin larvae in the autumn/winter.

Foals
No evidence exists that Parascaris equorum resistance has recently been assessed in the UK; however, clinicians working on stud farms consider P equorum resistance to macrocyclic lactones (both ivermectin and moxidectin) to be common and, in some regions, ubiquitous.
Fenbendazole and pyrantel appear to remain effective on most properties, although there are anecdotal reports of resistance to both drugs on UK stud farms.
FEC and FECRT should be used in foals to inform decision-making; however, prepatent infection is an important cause of disease, and strategic treatments also needs to be administered, particularly if stocking densities are high. Clinical signs in foals are typically seen from late summer or early autumn:
- Consideration should be given to treatment with fenbendazole at two to three months of age and again at four to five months of age in foals born early in the year.
- At seven to eight months of age, consideration should be given to the use of FEC to determine the need for treatment against P equorum (with fenbendazole), cyathostomins (ivermectin) or both (pyrantel).
- Moxidectin and praziquantel should be considered in autumn/winter with precise timing, dependent on risk and the age of the foal.
Strongyloides westeri is rarely a cause of disease and preventive treatment targeted against this parasite is not warranted, unless there is a history of disease on the property (meaning the routine treatment of mares prior to foaling is not indicated). Stud farms are at particular risk from anthelmintic resistance, and management practices need to change to prevent further development of resistance, to the point certain pastures cannot be used.
Stud farms also serve as a threat to the wider industry, as youngstock that harbour multi-resistant parasites disseminate widely by nationally and internationally introducing resistant parasites to other populations.
Conclusions
Veterinary surgeons should be looking to implement parasite control programmes on all of the premises that are under their care.
These programmes need to consider a number of factors that influence the risk of parasite-related disease with the ultimate aim being development of a strategy that prevents the development of clinical disease, while minimising the use of anthelmintics.
References
- Furtado T and Rendle D (2021). Creating environments for change: are there new ways to approach horse keeper behaviour in equine parasite control?, Veterinary Record 189(5): 197-199.
- Lightbody KL, Davis PJ and Austin CJ (2016). Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses, Veterinary Clinical Pathology 45(2): 335-346.
- Love S, Murphy D and Mellor D (1999). Pathogenicity of cyathostome infection, Veterinary Parasitology 85(2-3): 113-122.
- Lyons ET, Drudge JH, Tolliver SC, Swerczek TW and Collins SS (1989). Determination of the efficacy of pyrantel pamoate at the therapeutic dose rate against the tapeworm Anoplocephala perfoliata in equids using a modification of the critical test method, Veterinary Parasitology 31(1): 13-18.
- Morgan ER, Hetzel N, Povah C and Coles GC (2005). Prevalence and diagnosis of parasites of the stomach and small intestine in horses in south-west England, Veterinary Record 156(19): 597-600.
- Nielsen MK (2016). Equine tapeworm infections: Disease, diagnosis and control, Equine Veterinary Education 28(19): 388-395.
- Nielsen MK (2012). Sustainable equine parasite control: perspectives and research needs, Veterinary Parasitology 185(1): 32-44.
- Pittaway CE, Lawson AL, Coles GC and Wilson AD (2014). Systemic and mucosal IgE antibody responses of horses to infection with Anoplocephala perfoliata, Veterinary Parasitology 199(1-2): 32-41.
- Rendle D, Austin C, Bowen M, Cameron I, Furtado T, Hodgkinson J, McGorum BC and Matthews JB (2019). Equine de-worming: a consensus on current best practice, UK-Vet Equine 3: 1-14.
- Stratford CH, Lester HE, Morgan ER, Pickles KJ, Relf V, McGorum BC and Matthews JB (2014). A questionnaire study of equine gastrointestinal parasite control in Scotland, Equine Veterinary Journal 46(1): 25-31.