14 Dec 2021
Mélanie Perrier discusses treatment options for this cause of lameness in horses, as well as the role of rehabilitation and adjunctive therapies in successful management.

Image © 42beats / Adobe Stock
Degenerative joint disease (DJD) or OA is a common cause of lameness in horses, with surveys reporting up to 60% of lameness related to the condition.
It can affect different joints in the equine patient, with one common location being the tarsal joints (hocks).
OA is a degenerative process characterised by cartilage degradation, which is often accompanied by bone and soft tissue changes. It is recognised that the disease process may not only affect cartilage, but also the subchondral bone, articular capsule and periarticular tissue, including ligaments (Stewart and Kawcak, 2018).
In humans, two fundamental mechanisms have been described regarding risk factors for OA – “abnormal loading of normal cartilage” and “normal loading of abnormal cartilage”. This has been found to be similar in equine patients – especially racehorses – where not only can damage be afflicted to cartilage directly following concussion/cyclic fatigue damage, but also primary subchondral sclerosis and damage to the subchondral bone could result in further cartilage degeneration.
Similarly, inflammation of the joint – defined as synovitis – can also result in secondary cartilage damage following the release of inflammatory mediat
ors such as cytokines (Ross and Dyson, 2011).
In the equine tarsus, the tarsometatarsal and distal intertarsal joint are most often affected (hock spavin). Rotation and compression of the joint that occur at a canter are likely contributing to its development, together with the use of the horse.
Other inciting causes have been described, and include trauma (fracture, crushing of tarsal bones), excessive exercise, sepsis and osteochondrosis (Auer et al, 2019).
Horses affected with degenerative joint disease of the tarsi usually present with variable degrees of lameness, which is often bilateral and associated with decreased range of motion. Horses also often present with lack of hind end engagement, not being forward when ridden, decreased performance and “back problems”.
The equine tarsus is composed of five joints – tarsocrural, proximal intertarsal, distal intertarsal, tarsometatarsal, and talocalcaneal. Most of the movement is achieved in the tarsocrural joint, while the other joints are almost immobile. Synovial effusion is often noted in the tarsocrural joint, if affected, but may not be obvious for the distal tarsal joint where some remodelling may be palpated.
Affected horses may have a positive response to flexion test. Ultimately, diagnostic analgesia – and in particular response to intra-articular anaesthesia – will help localise the source of the lameness more precisely. Each joint should be anaesthetised individually.
Good and thorough knowledge of the anatomy and diffusion of the local anaesthetic is necessary. For example, concentrations of mepivacaine can be found in the centrodistal and tarsocrural joints following injection of the tarsometatarsal joint in 64% and 4% of limbs respectively. Similarly, after injection of the centrodistal joint, analgesic concentrations of mepivacaine were found in the tarsometatarsal and tarsocrural joints in 60% and 24% of limbs respectively. Also to be noted is that in cases with subchondral bone pain/extensive cartilage pathology, false negative results may be obtained and perineural anaesthesia may be preferred (peroneal tibial nerve block; Ross and Dyson, 2011).
Diagnostic imaging has an important role in making the final diagnosis of OA. Historically, radiographs (Figure 1) have been – and remain – the most common imaging modality used to diagnose arthritis; however, with the advance of new techniques, other modalities are now available.
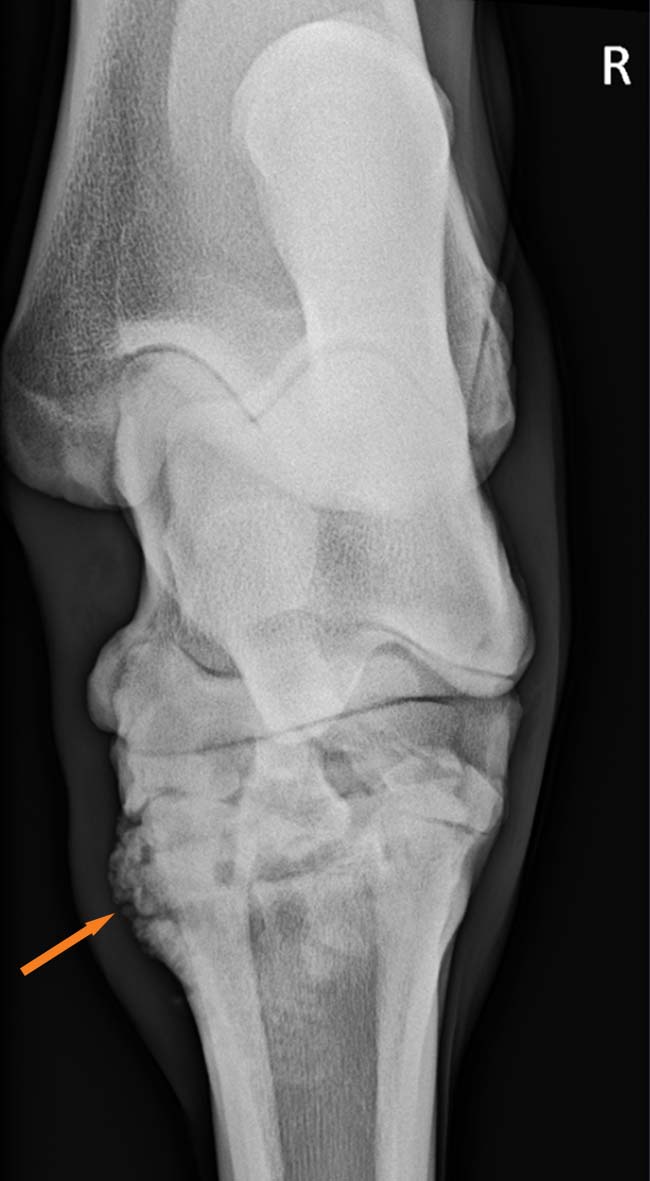
The main radiographic features of OA may include osteophyte formation, subchondral bone sclerosis and loss of joint space. Contrast radiographs may also be used in specific cases.
Ultrasound in conjunction to other modalities may be useful in evaluating the soft tissue structures surrounding the affected joint.
More recently, MRI and CT have also been used in the early diagnosis of OA, but are currently limited to the distal limb in most cases.
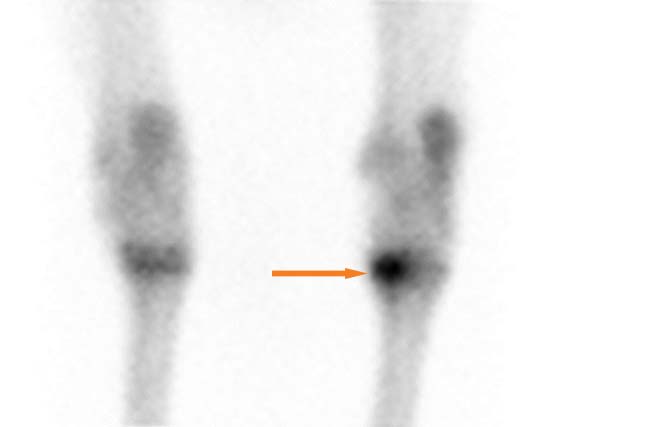
Finally, Nuclear scintigraphy (Figure 2) may be useful in diagnosing certain cases of OA, especially when performing locoregional anaesthesia may not be feasible or to localise early changes that may not be visible on radiographs (Auer et al, 2019).
With OA, treatments (Table 1) are mainly targeted at reducing the clinical signs associated with the disease, but some extend to preventing further degradation of the cartilage, bone and associated soft tissue (Goodrich and Nixon, 2006; McCarroll and McClure, 2000).
| Table 1. Summary of common non-surgical treatment options used in the management of tarsal OA | |||
|---|---|---|---|
| Dosing/frequency | Indications | Contraindications | |
| NSAIDs | |||
| Phenylbutazone | 2.2mg/kg to 4.4mg/kg SID to BID, IV/PO | • Fast-acting • Effective pain relief |
• GI ulceration • Renal disease |
| Firocoxib | • 0.3mg/kg loading • 0.1mg/kg SID, IV/PO |
Long-term use | Do not combine with other NSAIDs |
| Intra-articular steroids | |||
| Betamethasone | 4mg to 14mg/joint | Fast onset of action | Shorter acting |
| Methylprednisolone | 20mg to 80mg/joint | Less likely to cause laminitis? | • Harmful to cartilage? • Dystrophic soft tissue mineralisation • Avoid high motion joint |
| Triamcinolone | • 3mg to 10mg/joint • 18mg or less/horse |
• Chondroprotective • High motion joints |
May be more likely to cause laminitis? |
| Other intra-articular | |||
| Hyaluronic acid | 11mg to 22mg/joint | Chondroprotective effects | Post-injection reaction/flare |
| Platelet-rich plasma | 2ml to 6ml/joint | Available in field | • Variable response • Can cause reaction |
| Mesenchymal stem cells | 10 million to 20 million/horse | • Regenerative properties • Some positive results on horses with soft tissue injury |
• No significant effect on naturally occurring OA • Cost • Post-injection reactions • Do not use with antibiotics |
| Polyacrylamide gel | 2ml into affected joint | • Long-lasting • Reduced laminitis risks |
Cost |
| Other therapies | |||
| Shockwave | • 1,500 to 2,000 pulse/joint every 10 to 14 days for three treatments • Single treatment as needed |
• Non-invasive • Well tolerated |
Not within five days of competition |
| Bisphosphonates | |||
| Tiludronic acid | 1mg/kg IV in 10L fluids | Improved lameness score | Can cause colic symptoms |
| Clodronic acid | 1.8mg/kg IM | Ease of administration | Can cause colic signs, reaction at injection site |
| Chemical arthrodesis | |||
| Ethyl alcohol | 3ml of 70% ethyl alcohol | Relative ease of procedure (but necessity for contrast radiographs) | • Mild soft tissue swelling at injection site • Communication with proximal intertarsal joint |
| Monoiodoacetate | • 100mg in 2ml sterile saline • Three injections at zero, three and six weeks |
Evidence of bony bridging | • Severe lameness • Soft tissue reaction • Inconsistent results |
| KEY: SID = once daily; BID = twice daily; PO = orally; | |||
For the distal tarsal joints, joint stability and pain relief may be obtained by chemical or surgical fusion.
The ideal treatment would be able to provide pain relief while also producing disease modifying effects.
Nutraceuticals have been used in veterinary medicine for quite some time with mixed scientific evidence, but still to this day represent an important part of the veterinary market.
Nutraceuticals refer to compounds that are neither nutrients nor pharmaceuticals. They can include products such as dietary supplements, nutrients and functional foods.
While popular with owners, the scientific evidence regarding their efficacy in management strategy and treating joint disease is poor overall.
Among the available products, glucosamine-based products are quite popular. In one article looking at science-based evidence for glucosamine-based products used in equine medicine, a total of 15 publications were reviewed. Among those, only three met the quality standard. The main limitation was most studies did not support the dose of product tested with objective experimental evidence, with most relying on the advice of the product manufacturer, which should be addressed with owners when selecting such products (Trumble, 2005; Pearson and Lindinger, 2009).
Systemic administration of NSAIDs remains at the forefront of management for most arthritis cases.
Over the years, various medications have been used. If phenylbutazone probably remains most commonly used, more cyclooxygenase (COX) type two selective molecules – such as firocoxib – have also been used with variable clinical efficacy.
The efficacy of NSAIDs relies on their anti-inflammatory properties, but also their modulation of pain through the COX inhibition pathway, while corticosteroids inhibit synthesis of arachidonic acid via phospholipase A2.
Among the COX inhibition pathway, COX-1 is known to have a protective role in the balance of normal physiological function of the gastrointestinal and renal systems, while COX-2 has mainly been associated with the inflammatory cascade.
Therefore, some efforts were made to develop some more COX 2 selective drugs. Among them, firocoxib has been studied for its use in managing equine OA.
In one study, 253 horses diagnosed with arthritis were treated either with phenylbutazone or firocoxib. In that study, they were found to both have a similar effect on clinical improvement in lameness for horses with naturally occurring OA and navicular disease 7 and 14 days after administration (Doucet et al, 2008; Donnell and Frisbie, 2014).
Other NSAIDs commonly used include meloxicam, ketoprofen and carprofen, with some reports that carprofen may delay the progression of OA and have symptom‑modifying effects in the dog, but reports on the horse remain limited.
Tiludronic acid and clodronic acid are bisphosphonates that have been used for their potent inhibition of bone resorption mainly by inhibiting osteoclasts. They are also believed to have some anti-inflammatory properties through nitric oxide and cytokine release. Finally, they have been shown to inhibit the secretion of cartilage-degrading enzymes induced by interleukin-1 in the chondrocyte or the synovial cell (McLellan, 2017).
One publication investigated using tiludronic acid at 1mg/kg IV for the treatment of hock spavin in 42 of 87 horses; the remaining horses receiving a placebo. At day 60 the lameness score was lower (2.6 +/- 1.7) for the tiludronate group compared to the placebo group (3.3 +/- 2.0). Significant improvement was also seen in lameness from day 60 to day 120 for tiludronate‑treated horses (Gough et al, 2010).
Several intra‑articular medications are available on the market. Most of these medications are aimed at reducing the inflammation and, therefore, the pain associated with the condition.
Historically, corticosteroids (Vandeweerd et al, 2015) – used in combination with or without hyaluronic acid – has been the core of intra‑articular medication.
For the distal tarsal joint, methylprednisolone has been used since it is considered a low motion joint, while triamcinolone may be preferred in the tarsocrural joint.
Laminitis is always a side effect that owners should be warned about when using corticosteroids.
Recently, the use of so‑called non-steroidal intra‑articular therapies – such as biological and synthetic products – has gained increasing interest on the equine market. Among products commonly used, autologous‑conditioned plasma and serum, autologous‑conditioned solution, cellular therapeutics and polyacrylamide gel were found to be the most popular with equine practitioners in one study (Caron, 2005; Broeckx et al, 2019; Camargo Garbin and Morris, 2021).
Polyacrylamide gel is a non‑immunogenic biocompatible polymer gel consisting of 97.5% sterile water and 2.5% cross-linked polyacrylamide (de Clifford et al, 2019; Tnibar et al, 2014; 2015). It exerts its effect through integration within the soft tissues over time, and through a combination of vessel ingrowth and molecular water exchange.
In one study, polyacrylamide gel was found to be safe, and significantly alleviate lameness and joint effusion in osteoarthritic joints (de Souza et al, 2020). In this study, polyacrylamide gel treatment lasted at least 24 months and was concluded to be a promising new treatment for OA in horses.
With the recent advances in equine rehabilitation and the increasing requirement for newer management options for degenerative joint disease, various modalities are now available on the market for treatment of arthritis (Contino, 2018).
Most of those modalities remain poorly regulated and their efficacy show variable scientific evidence, but they represent a continuously growing and attractive market for horse owners.
When considering hock arthritis, a lot of the modalities used will be targeted at muscle conditioning of the upper limb in conjunction to some of the medical treatments already discussed. They are also used for their analgesic effect.
Shockwave therapy has been used with some evidence for treating arthritis of the tarsal joints (Revenaugh, 2005). Shockwave uses pressure waves to create microtrauma and neovascularisation.
It also provides analgesic effects through four main physical phases:
In a study looking at using shockwave for the treatment of hock arthritis in 74 horses, improvement was seen in one grade of lameness in 80% of the cases, which resulted in 18% of the cases becoming sound. This study suggests that shockwave could be used in conjunction with other treatment to manage OA (McCarroll and McClure, 2000).
Laser has limited evidence in the equine literature to this day, but publications on the small animal side of the veterinary medicine (Barger et al, 2020) and the human field show promise for its use.
Laser – also known as photobiomodulation therapy – has been proven to increase acetylcholine production, which is an inhibitory neurotransmitter, and to decrease bradykinins production, which, in turn, significantly increases pain threshold required for transmission sensory nerve input. Laser also may have some direct analgesic effect on nerves.
In one study (Barale et al, 2020) looking at using low‑level laser therapy on 17 dogs diagnosed with OA, the laser provided some pain relief and improved function in treated animals, making it a promising tool for future use. Further studies looking at protocols for use in equine medicine is warranted.
Chemical arthrodesis using ethyl alcohol has been used mainly in the pastern and distal tarsal joints (Carmalt et al, 2012).
Ethyl alcohol acts through non-selective protein denaturation, and cell precipitation and dehydration, and functions as a neurolytic agent resulting in a sensory innervation blockade at the intra-articular level.
When using ethyl alcohol to provide chemical fusion of the tarsometatarsal joint, it is advised to perform a contrast arthrogram of the joint prior to injection to rule out communication with the proximal intertarsal joint.
In one study (Lamas et al, 2012) with long‑term follow‑up, 52% of the horses were improved while 19% worsened following injection. This remains a viable option for horses that did not respond to other form of therapies and for whom the owners are not willing to consider surgical treatment (Bell et al, 2009).
Monoiodoacetate (MIA) is another substance that has been used historically for chemical arthrodesis of joint in horses.
MIA is responsible for articular cartilage destruction by inhibiting chondrocyte metabolism to produce chondrocyte death. In one study, 40% to 90% of the animals had successful outcomes after MIA injection and 97% of the horses presented bone bridging the joints at 12 months (Bohanon et al, 1991).
The use of this chemical has been limited due to the inconsistent response it provides, and some reported severe soft tissue reaction and associated discomfort for the patient.
More invasive techniques for fusing the affected tarsometatarsal and distal intertarsal joints have also been described and include drilling of the joint with or without concurrent use of laser.
In one study comparing standard drilling, MIA injection and laser‑assisted arthrodesis, laser surgery produced the least postoperative morbidity (Zubrod et al, 2005).
In more severe cases, internal fixation using plate and screws may be necessary to achieve successful fusion. One study (Biedrzycki et al, 2016) compared surgical drilling, medially to laterally placed kerf-cut cylinders (MLKCs), dorsally applied kerf-cut cylinder (DKC), and a dorsomedially applied locking compression plate (DMLCP). It concluded that standard drilling may reduce tarsal stability in horses without clinical OA, compared with stability with no intervention, while the DMLCP and DKC approaches significantly enhanced stability.
For OA of the talocalcaneal joint, arthrodesis using three cortical screws implanted in lag fashion across the joint has yielded some good results (Pauwels et al, 2005; Smith et al, 2005; Figure 3).
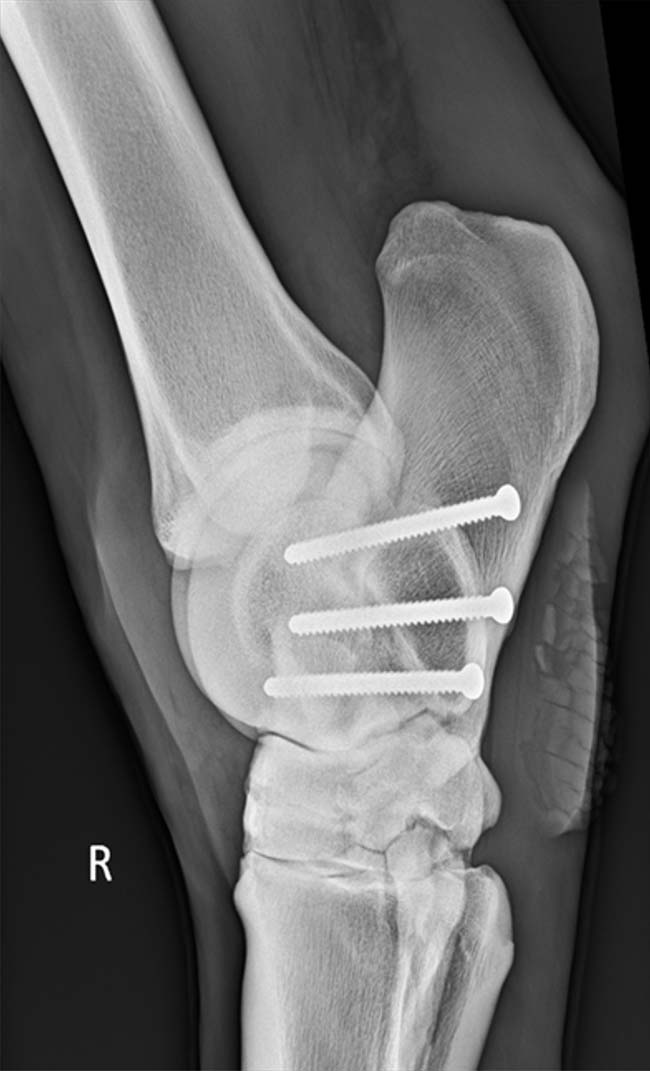
Overall surgical arthrodesis has been reported to be as high as 85% in some reports (Archer et al, 1988; Edwards, 1982).
Cunean tenectomy, which consists in standing removal of a small 4cm section of the cunean tendon, has also been reported as a surgical option for arthritis of the tarsus, resulting in decreased rotational forces on the joint. The use of this technique is controversial and may only relieve pain in horses where the amount of new bone formation under the tendon may be the cause of pain (Auer et al, 2019).
Newer treatment options are being evaluated in the human side. Among those, treatment targeting cartilage with matrix metalloproteinase inhibitors and bone morphogenetic protein 7 are under way, with some positive results regarding pain improvement.
Several treatments specifically aimed at targeting inflammatory mediators and pathways are also under evaluation. In particular, novel gene therapy using recombinant adenovirus encoding interlukin‑1 receptors are under way.
Finally, treatments addressing pain through anti-nerve growth factor strategy and drugs targeting opioid receptors are being evaluated (Grässel and Muschter, 2020).
Arthritis remains the most common cause of lameness in the horse – and if newer treatments are available and being evaluated, the core of arthritis management remains systemic anti‑inflammatory and intra-articular medication.
With the development of equine rehabilitation and of adjunctive therapy, it is hoped these modalities will be used as adjunctive therapy to help manage OA successfully.
Some drugs mentioned in this article are used under the cascade.