5 May 2023
Ending the nightmare of strangles
Andrew S Waller outlines how this disease is spread around liveries and how veterinarians can help to eradicate it using new medicines.

Strangles remains the most frequently identified infectious disease of horses, responsible for the suffering and death of countless animals.
The causative agent, a Gram-positive bacteria called Streptococcus equi subspecies equi, is believed to have plagued populations of horses for hundreds – if not thousands – of years1.
However, analysis of the genome sequences of S equi recovered from 19 different countries found that the “BAPS2” group of strains, which is responsible for more than 95% of disease in horses residing in the UK today, originated as recently as the 1990s2.
The relatively rapid emergence and spread of contemporary strains of S equi reflect the unique lifestyle of this pathogen and its ability to exploit the anatomy of the horse, equine responses to infection, and the mobility of horses that facilitates transmission to previously unaffected herds.
Pathogenesis and clinical signs
Horses become infected following entry of S equi into the nasopharynx and oropharynx through direct nose-to-nose contact with an infected horse, the consumption of contaminated food or water, or contact with fomites such as contaminated riding equipment, stalls, clothing and humans (Figure 1).
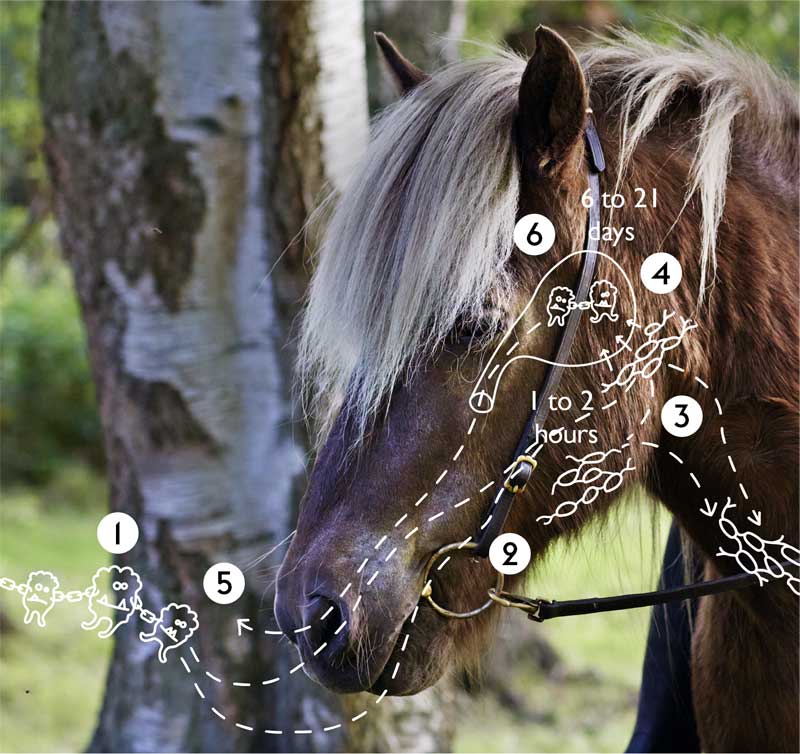
Once within the horse, S equi rapidly invades the lymph nodes of the head and neck, where it can be detected within a few hours3. Such a rapid translocation to the lymph nodes may actually be mediated by immune cells that engulf, but fail to kill, S equi during this critical early phase of infection.
The death of these equine immune cells enables release of S equi into its target site: the equine lymph node. Here, S equi produces an arsenal of toxins and immune mediators to evade and misdirect the equine immune response, resulting in damage to host tissue and the release of nutrients that facilitate bacterial growth, and the formation of abscesses4.
The first clinical sign of S equi infection, a raised body temperature equal to or more than 38.5°C, reflects the growing inflammatory response, and usually precedes shedding of the bacteria by two to three days. Therefore, the monitoring of body temperature is the most effective tool with which to identify infected horses before they have the opportunity to transmit S equi to in-contact animals.
Over the following days, abscesses in the lymph nodes of affected horses continue to grow and may achieve a size so great as to restrict the airway, leading to the death of some animals and providing an explanation of the naming of this disease (Figure 2). Eventually, after a period of between 6 and 21 days, the abscesses burst, releasing highly infectious pus.
Studies in which horses were challenged by spraying S equi into the nasopharynx showed that as few as 1,000 bacterial cells were sufficient to establish infection, but abscesses can contain billions of S equi cells. Therefore, a single drop of pus released from an abscessed lymph node has the theoretical potential to infect hundreds of naive horses, highlighting the importance of early diagnosis so cases can be isolated to minimise the exposure of other animals to S equi.
Profuse mucopurulent nasal discharge may be apparent in affected horses as their lymph node abscesses drain, and pus may be released from abscesses that burst through the horse’s skin. However, in some horses, abscesses form in lymph nodes distant from the head and neck, leading to the development of a serious condition known as “bastard strangles”, which is often fatal.
The production of excessive levels of antibodies against the immunodominant SeM protein of S equi has been linked with cases of purpura haemorrhagica characterised by vascular leakage and oedema, which can also be fatal5.
In some outbreaks, in the absence of effective infection control and where exposure to S equi is high, all resident horses may develop strangles and up to 10% of affected animals may die; for example, an ongoing outbreak in unvaccinated European sport horses had affected more than 100 animals and killed 6 horses in the period up to March 2023.
Persistent infections
In the majority of cases, abscess material drains from affected horses and the infection resolves. However, despite recovering from clinical disease, approximately 10% of horses fail to eliminate S equi from their guttural pouches, where the organism can persist for several years.
These healthy, recovered, “carrier” horses intermittently shed S equi into the environment, enabling transmission to new horses and the initiation of further cases of disease.
Therefore, the prevention of lymph node abscesses, and the identification and treatment of carriers is critical towards reducing the prevalence, cost and impact of this devastating disease.
Strangles surveillance
The Surveillance of Equine Strangles project – supported by The Horse Trust and based at the RVC – reported that 347 horses were diagnosed with S equi infection in 2022 following the testing of samples collected by 123 different veterinary practices across 63 different regions of the UK (Figure 3).
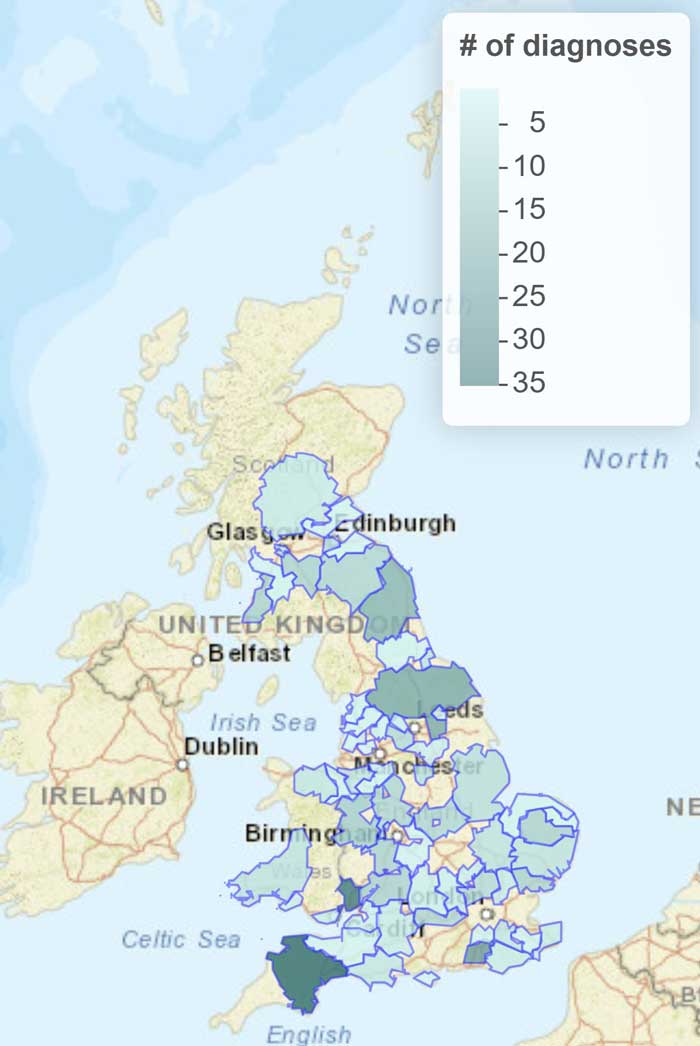
Diagnoses occurred throughout the year, with the highest number occurring in August and the lowest in November. Sport horses were the breed most frequently diagnosed with strangles during 2022, but no horse breeds are naturally resistant to infection with S equi and any farm with horses can be affected by this disease.
To help improve awareness of current outbreaks, veterinarians can now receive monthly updates on strangles diagnoses via the Tell-Tail alert service, which is supported by Boehringer Ingelheim.
New research at the RVC has provided evidence of transmission of genetically related strains of S equi to premises surrounding outbreaks (McGlennon et al, unpublished data).
Furthermore, “super-spreader” transmission events – akin to the outbreak of neurological disease caused by equine herpesvirus-1 in Valencia during 2021 – are also being identified through the analysis of genome sequencing data using techniques developed during the COVID-19 pandemic (McGlennon et al, unpublished data).
Several outbreaks of strangles are associated with the return of horses following competitions or the arrival of new horses. New horses might have been infected prior to or during transport, and were incubating the disease on arrival, or they might have been persistently infected following recovery from a previous episode of disease.
The quarantine of new horses for three weeks minimises the risk of exposure of resident horses to pathogens. Similarly, keeping returning competition horses separate from resident horses can reduce the risk of disease transmission.
Daily temperature checks are invaluable to identify early signs of disease, enabling veterinarians to control the spread of infection. Furthermore, the vaccination of horses before attending events, or the arrival of a new horse, has the potential to greatly reduce the number of animals affected and the severity of any cases of disease.
The fusion-protein based vaccine, Strangvac, was launched in the UK during 2022 by Dechra, with Intervacc being the market authorisation holder. The vaccine contains eight important and highly conserved proteins that normally help S equi to attach to equine tissue, misdirect the equine immune response and cause disease6.
Importantly, the vaccine contains no live S equi and is administered by IM injection (Figure 4). The vaccine does not interfere with any of the available diagnostic tests for strangles7. Furthermore, it does not contain the SeM protein, so avoiding the production of anti‑SeM antibodies associated with cases of purpura haemorrhagica5.

These features enable the vaccine to generate productive immune responses in horses without interfering with their normal management protocols, and the vaccine has been lauded by world leaders in the prevention of equine infectious diseases, including Richard Newton, director of epidemiology and disease surveillance at the University of Cambridge Veterinary School, and Gittan Gröndahl, deputy state veterinary officer at the Swedish National Veterinary Institute.
Their expectation is that the wider use of vaccination, alongside current measures to control strangles, should ultimately reduce the number of strangles outbreaks to a level equivalent to equine influenza, providing tremendous benefits to horses and equestrian communities.
Early reports of the use of the new vaccine in the field have been extremely promising and it has already been used successfully by veterinarians across Europe to protect horses (Figure 5).
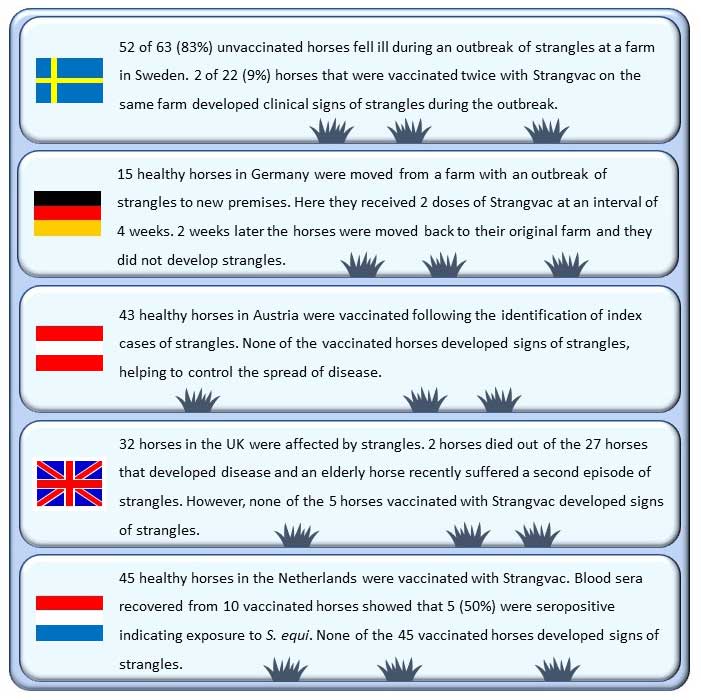
Ideally, horses should be vaccinated before an outbreak occurs, so more time is available for immunity to develop. An initial course of two doses four weeks apart can be followed by revaccination every six months to maintain immunological memory, and trials demonstrated that three doses can protect up to 94% of horses7.
In outbreak situations, it is important that horses with signs of strangles are isolated as quickly as possible in a “red” group, regardless of vaccination status.
The monitoring of the body temperatures of “amber” group horses, which had contact with cases, and “green” group horses, which did not have contact with cases, is a valuable tool to identify horses in the early stages of disease, so that they can also be isolated in the red group.
Only healthy horses that are not incubating the disease should be vaccinated (that is, those in the green group). Should the temperature of amber group horses remain normal over several weeks, and the attending veterinarian considers them to not be incubating the disease (green group), then they could also be vaccinated towards increasing the level of immunity within the herd.
Healthy vaccinated and non-vaccinated horses can be screened four weeks after the resolution of the final clinical case using the strangles iELISA to identify horses that were exposed to S equi during the outbreak, which can then be investigated further to identify and resolve persistent infections, and to clear S equi from the premises.
Continued vaccination of horses every six months thereafter will help to minimise the risk of a new incursion of strangles and maintain a state of readiness to maximise immunity against S equi, should another case occur.
Conclusions
Strangles remains a highly prevalent and devastating disease that is endemic within the UK horse population.
Emerging evidence supports the use of a new fusion-protein based in healthy horses as a tool to reduce the transmission of S equi, helping to prevent, contain and resolve outbreaks.
However, it is important to remember that vaccination alone will not control an outbreak, and it should be used in combination with daily biosecurity, temperature checking, isolation of affected horses, and the control of movement on and off the premises.
As the use of vaccines and immunity across horse populations increases, it is hoped the prevalence of strangles will decline so that fewer horses, owners and veterinarians are required to endure the nightmare of a strangles outbreak.
References
- Harris SR et al (2015). Genome specialization and decay of the strangles pathogen, Streptococcus equi, is driven by persistent infection, Genome Res 25(9): 1,360-1,371.
- Mitchell C et al (2021). Globetrotting strangles: the unbridled national and international transmission of Streptococcus equi between horses, Microb Genom 7(3): mgen000528.
- Timoney JF and Kumar P (2008). Early pathogenesis of equine Streptococcus equi infection (strangles), Equine Vet J 40(7): 637-642.
- Holden MTG et al (2009). Genomic evidence for the evolution of Streptococcus equi: host restriction, increased virulence, and genetic exchange with human pathogens, PLOS Pathog 5(3): e1000346.
- Pusterla N et al (2003). Purpura haemorrhagica in 53 horses, Vet Rec 153(4): 118-121.
- Frosth S et al (2023). Conservation of vaccine antigen sequences encoded by sequenced strains of Streptococcus equi subsp. equi, Equine Vet J 55(1): 92-101.
- Robinson C et al (2020). Intramuscular vaccination with Strangvac is safe and induces protection against equine strangles caused by Streptococcus equi, Vaccine 38(31): 4,861-4,868.
