15 Mar 2022
Endocrinopathic laminitis: cause identification and management
Nicola Menzies-Gow describes this most frequently seen form of laminitis and its association with two common endocrine disorders – equine metabolic syndrome and pituitary pars intermedia dysfunction.

Image © 42beats / Adobe Stock
Endocrinopathic laminitis is the most common form of laminitis and encompasses laminitis associated with insulin dysregulation (ID). Therefore, it includes laminitis associated with the two common endocrine disorders equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID).
ID manifests in three forms and tests are available to detect each of these forms, including measurement of basal serum insulin concentrations, the oral sugar test and the insulin tolerance test. PPID is detected by measurement of basal adrenocorticotropic hormone (ACTH) and/or the ACTH response to thyrotropin-releasing hormone.
ID, regardless of whether it is associated with EMS or PPID, is managed through a combination of diet and exercise (if no active laminitis exists). Pharmacologic agents are used in the short term in animals that are refractory to management changes alone. PPID is managed through treatment with the dopamine agonist pergolide.
Three types of laminitis exist – namely, sepsis-associated, endocrinopathic and supporting limb laminitis. Endocrinopathic laminitis is the commonest form, accounting for up to 90% of cases.
It encompasses laminitis associated with insulin dysregulation (ID) and includes laminitis associated with the two common endocrine disorders: equine metabolic syndrome (EMS) and pituitary pars intermedia dysfunction (PPID).
Identification of causes of endocrinopathic laminitis
ID is the central feature of EMS and the subset of animals with PPID that develop laminitis appear to have ID. Therefore, investigation of endocrinopathic causes of laminitis requires identification of ID in suspect EMS and PPID cases, and detection of PPID in suspect PPID cases.
Altered circulating concentrations of adipose tissue-derived hormones (adipokines) such as adiponectin are found in a proportion of animals with EMS and so can provide additional information.
Identification of ID
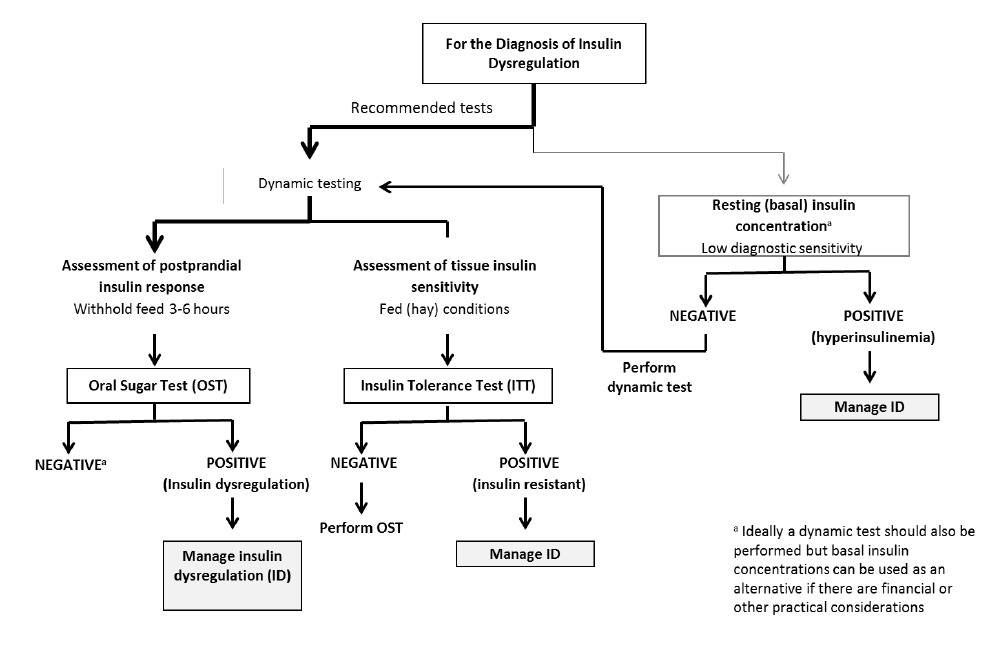
ID manifests in three forms – namely, hyperinsulinaemia, an excessive insulin response to oral carbohydrate and peripheral (tissue) insulin resistance. Any combination of these manifestations may occur in an individual animal; therefore, it is important to test for all three (Figure 1).
Basal insulin concentrations
Hyperinsulinaemia is detected by measuring basal insulin concentrations in a single blood sample collected with the horse in the fed state (hay or pasture, but not grain). See Table 1 for interpretation guidance.
| Table 1. Basal insulin concentrations (produced by the Equine Endocrinology Group) | ||
|---|---|---|
| Results | Interpretation | Recommendation |
| <20μU/ml (radioimmunoassay [RIA] and Immulite 1000) <31μU/ml (Immulite 2000 XPi) |
Non-diagnostic | Dynamic test recommended to better assess |
| 20μU/ml to 50μU/ml (RIA and Immulite 1000) 30μU/ml to 75μU/ml (Immulite 2000 XPi) |
ID suspect if consistent clinical signs | |
| >50μU/ml (RIA and Immulite 1000) <75μU/ml (Immulite 2000 XPi) |
Insulin dysregulation | Proceed with ID management |
Oral sugar test
An oral sugar test (OST) is a dynamic test recommended to detect an excessive insulin response to carbohydrate. The OST is preferred because the insulin concentrations measured reflect a more complete sequence of events including digestion and absorption of sugars, incretin hormone responses and secretion of insulin from the pancreas.
Advantages of the test include the ready availability of commercial corn syrup, ease of administering corn syrup and the test’s assessment of insulin responses to ingested sugars.
Disadvantages include the requirement recommendation for horses to ideally be fasted for three to six hours prior to testing; however, fasting conditions are often achieved by the owner leaving one flake/slice of hay with the horse before midnight and then the test is performed the following morning. Alternatively, the test can be performed while the horse remains at pasture.
In situations where horses are resistant to oral administration, the corn syrup can be mixed with a small amount of low-glycaemic feed (for example, chaff). Oral glucose dextrose powder can alternatively be used and should also be mixed with a small amount of low-glycaemic feed. See Table 2 for test details and interpretation guidance.
| Table 2. Dynamic tests (produced by the Equine Endocrinology Group) | ||
|---|---|---|
| Postprandial insulin response | Insulin sensitivity | |
| Oral sugar test | Insulin tolerance test | |
| Procedure | Fast 3 to 6 hours Administer 0.45ml/kg corn syrup orally via dose syringe Collect blood at 60 and/or 90 minutes Measure insulin and glucose |
Fed (pasture or hay) state. Do not fast Collect blood at time 0 and administer 0.10IU/kg regular (soluble) insulin Collect blood at 30 minutes Measure glucose Feed meal immediately after last sample |
| Interpretation | >65μU/ml for 0.45ml/kg test and radioimmunoassay >63μU/ml positive for 0.45ml/kg test and Immulite 2000 XPi Assess baseline (fasting) glucose concentration to detect diabetes mellitus (rare) |
<50% decrease in blood glucose concentrations from baseline is consistent with insulin resistance |
| Alternative tests | In-feed oral glucose tolerance test | Combined glucose-insulin test |
| Note that hypoglycaemia is a risk associated with this test. Provide feed after collecting the second blood sample. In rare cases, dextrose solution has been administered intravenously to address hypoglycaemia. | ||
Insulin tolerance test
The ITT is recommended to detect peripheral (tissue) insulin resistance. Advantages of the ITT are that this test does not require pre-test fasting and blood glucose concentrations can be measured with a glucometer so preliminary results are available on the farm.
Disadvantages include the cost of purchasing the insulin and the very small risk of clinical hypoglycaemia developing. See Table 2 for test details and interpretation guidance.
Identification of PPID
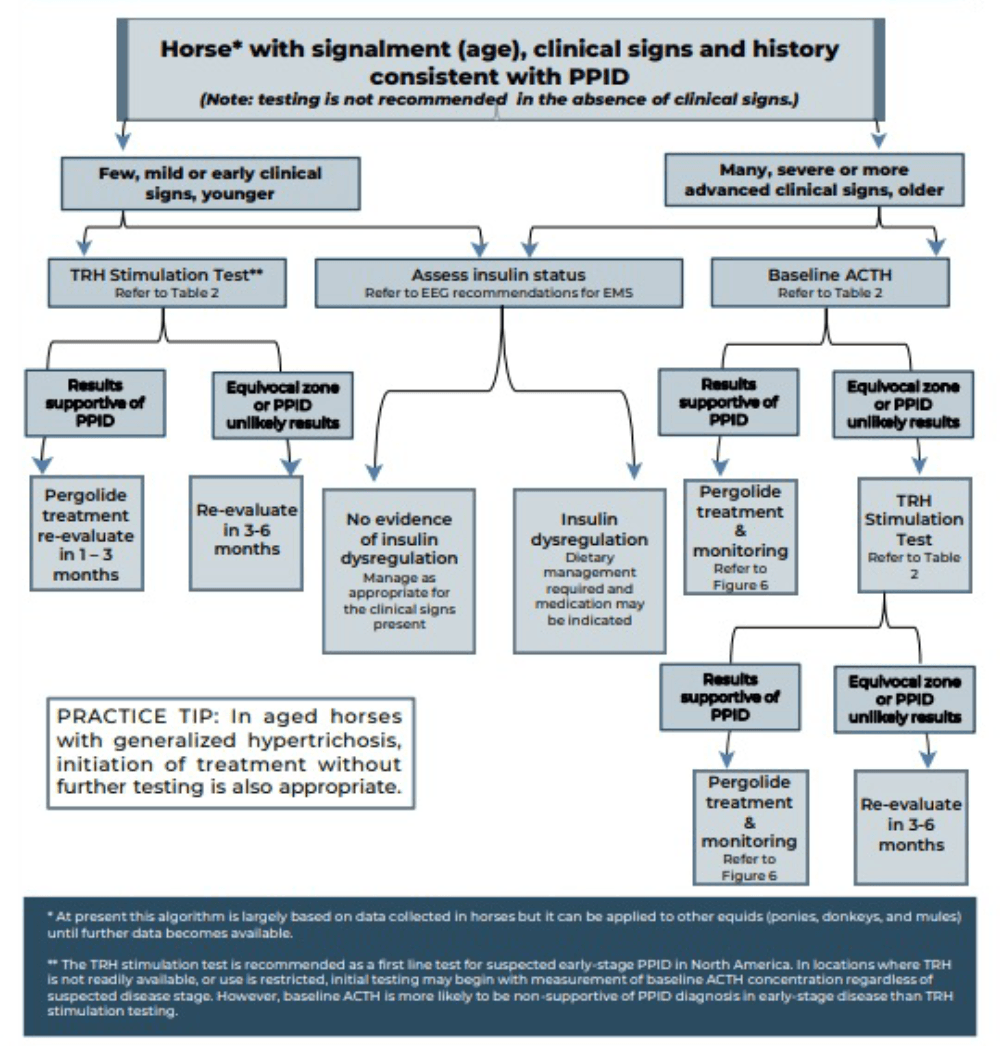
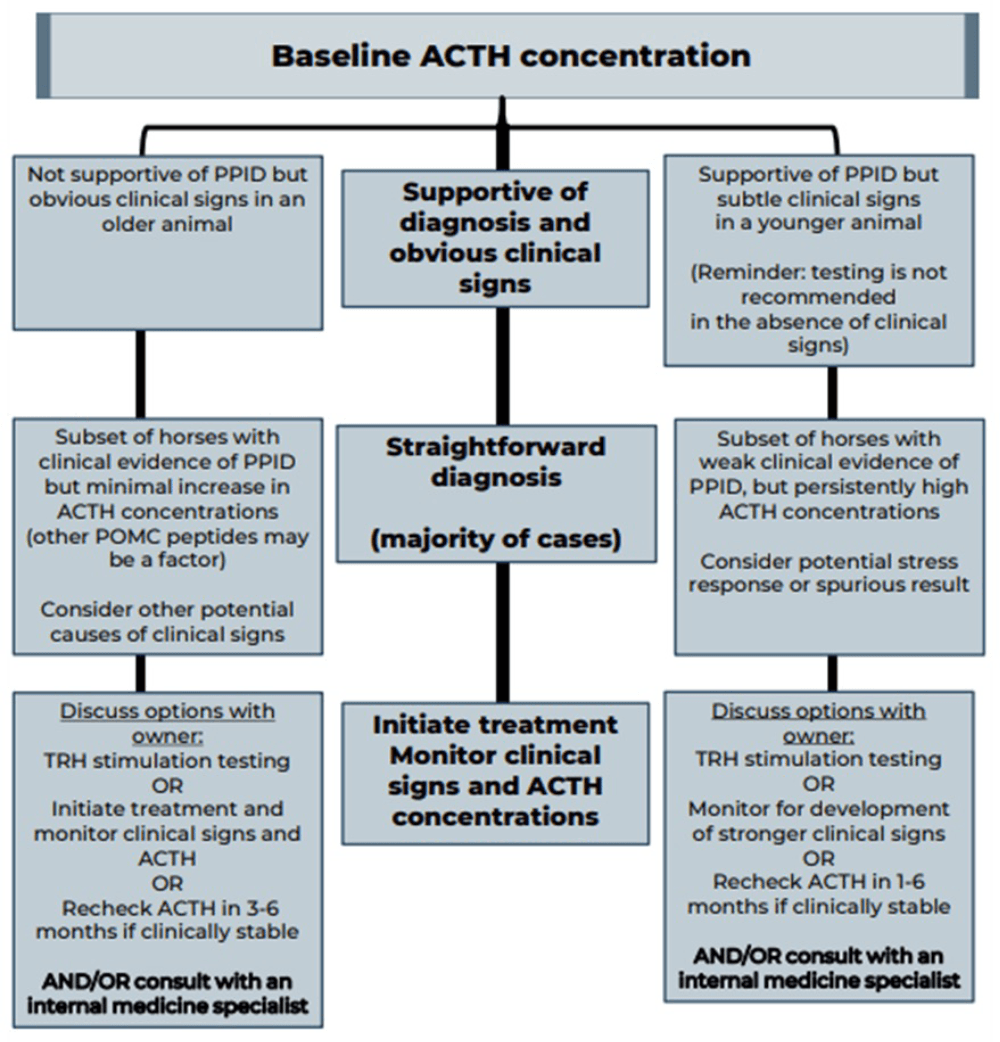
Measurement of basal adrenocorticotropic hormone (ACTH) concentration is a practical test for diagnosis of PPID, although variation in individuals and over time results in some overlap in ACTH concentrations between normal and PPID populations.
As ACTH concentrations increase in the autumn in healthy and PPID animals, interpretation of ACTH testing for PPID diagnosis requires seasonally adjusted values. When basal ACTH concentrations are in the equivocal zone, veterinarians should use clinical information such as age and severity of signs to decide whether to monitor and re-test, follow up with dynamic thyrotropin-releasing hormone (TRH) stimulation testing, or in some cases, to treat.
Because the basal ACTH concentration is more likely to fall in the equivocal zone in early-stage disease, the TRH stimulation test may also be an appropriate first line test in many cases. See Figures 2 and 3 and Table 3 for test details and interpretation guidance.
| Table 3. Basal adrenocorticotropic hormone (ACTH) concentration and thyrotropin-releasing hormone (TRH) stimulation test (produced by the Equine Endocrinology Group [EEG]) | ||||
|---|---|---|---|---|
| Seasonal interpretation of results* | Pituitary pars intermedia dysfunction (PPID) unlikely | Equivocal* Requires strong clinical signs, re-testing or TRH stim to confirm diagnosis |
PPID likely | |
| Baseline ACTH or TRH time 0 (pg/ml) *See important note below |
Dec-Jun | <15* | 15-40* | >40 |
| Jul and Nov | <15* | 15-50* | >50 | |
| Aug | <20* | 20-75* | >75 | |
| Sep-Oct | <30* | 30-90* | >90 | |
| 10 minutes after TRH (pg/ml) | Jan-Jun | <100 | 100-200 | >200 |
| Jul-Dec | <100 | TRH stimulation testing can only be used to identify negative cases in these months due to many false positives | ||
Important notes
|
||||
Measurement of adipokine concentrations
Concentrations of the adipokine adiponectin are decreased in a proportion of animals with EMS and associated with an increased risk of future endocrinopathic laminitis independent of insulin concentration. Therefore, measurement provides additional information relating to laminitis risk.
Managing endocrinopathic laminitis
Management of a case of endocrinopathic laminitis involves managing the underlying endocrinopathy, as well as the laminitis itself.
Managing ID
The management of ID consists of dietary modification, exercise and use of pharmacologic agents.
Diet
Dietary modification recommendations depend on whether the animal is obese or lean.
Obese animals
Obesity is managed primarily via energy restriction through limiting intake. An ideal target for weight loss is 0.5% to 1% of body mass (BM) weekly.
A daily allowance of 1.25% to 1.5% of actual BM as dry matter intake (DMI), or 1.4% to 1.7% of actual BM as fed, is widely recommended. In horses with weight loss resistance, a further forage restriction to 1% BM as DMI or 1.15% BM as fed may be considered if appropriately monitored. Grains or cereal-based feeds should be excluded due to their high content of non-structural carbohydrate (NSC) and high-fat feeds should be avoided due to their high energy content. Additionally, large amounts of fruit or vegetables, such as carrots, apples or treats with a high glycaemic load, should be avoided.
The nutrient composition of the forage should be determined where possible and hays with low NSC content (less than 10%) are recommended to limit post-prandial insulin responses. Soaking for one hour in warm water or six hours in cold water is advised to reduce the NSC content of the hay if necessary.
Straw is a cost-effective and low-energy forage that may be used as an alternative to hay alone. As forages can be low in protein, and mineral and vitamin leaching occurs after soaking, these nutrients must be balanced by low-calorie supplements to cover requirements.
During the initial 6 to 12 weeks of dietary restriction, pasture access should be prevented as even partial access is very difficult to quantify.
However, successful long-term management of EMS cases can still include some grazing, provided that the ID – especially assessed by the insulin response to oral carbohydrates or grazing – is under control and that grazing is also carefully controlled.
The use of dietary supplements such as cinnamon, magnesium and chromium to facilitate weight loss or to improve ID is popular, but their efficacy remains questionable or unproven.
Lean animals
Lean animals should be fed a low glycaemic diet to minimise the postprandial insulin response.
The diet should be based on forage with a low (ideally less than 10) NSC content and additional calories provided in the form of fat (for example, vegetable oil) and high-quality fibre such as beet pulp. A low-calorie vitamin, mineral and protein ration balancer should be fed as required.
Exercise
Exercise has been shown to improve insulin sensitivity in people; however, studies in horses have shown variable results. Exercise should only be considered in animals without current laminitis and should be gradually increased based on fitness.
The optimal exercise regime has yet to be determined; however, half-hour sessions of exercise that raise heart rate to more than 150 beats per minute performed five days per week are recommended.
Pharmacologic agents
Metformin is the most common drug that has previously been prescribed for managing ID in horses.
Doses range from 15mg/kg to 30mg/kg given two to three times daily by mouth and it is recommended that the drug is ideally administered 30 to 60 minutes prior to feeding.
However, the oral bioavailability is extremely poor, and the drug does not appear to improve insulin sensitivity in equids. However, it had a beneficial effect through blunting of postprandial increases in glucose and insulin concentrations in a single study.
Levothyroxine (0.1mg/kg to 0.15mg/kg by mouth once a day) may be used in obese animals to accelerate weight loss through increasing the metabolic rate, but must be used in conjunction with diet and exercise changes as reported side effects include polyphagia.
The dose should be gradually reduced and then treatment discontinued after the weight loss has been achieved or after three to six months of therapy.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors may prove useful in the future once more scientific evidence is available. Velagliflozin is a SGLT2 inhibitor that reduces renal glucose reabsorption, promotes glucosuria and, consequently, decreases blood glucose and insulin concentrations in humans.
It has also been shown to reduce hyperinsulinaemia and prevent laminitis in insulin-dysregulated ponies fed a challenge diet high in non-structural carbohydrates in a single study with small numbers of animals; however, it is not currently commercially available.
Canagliflozin was reported to be effective at decreasing the insulin response to an oral sugar test in a preliminary US study using six horses and ertugliflozin has been shown to have a similar effect in a study using 11 animals.
Lipid mobilisation is stimulated in many horses treated with SGLT2 inhibitors and hypertriglyceridaemia may develop as a consequence. Therefore, horses with marked hypertriglyceridaemia should not be treated with these drugs.
It should be remembered that none of these drugs are licensed for use in horses and each is used in accordance with the cascade.
Managing PPID
PPID is a slowly progressive, lifelong condition. The aim of treatment is not to cure the condition, but to increase the quality of life through reducing the clinical signs, including those that have the potential to be life threatening.
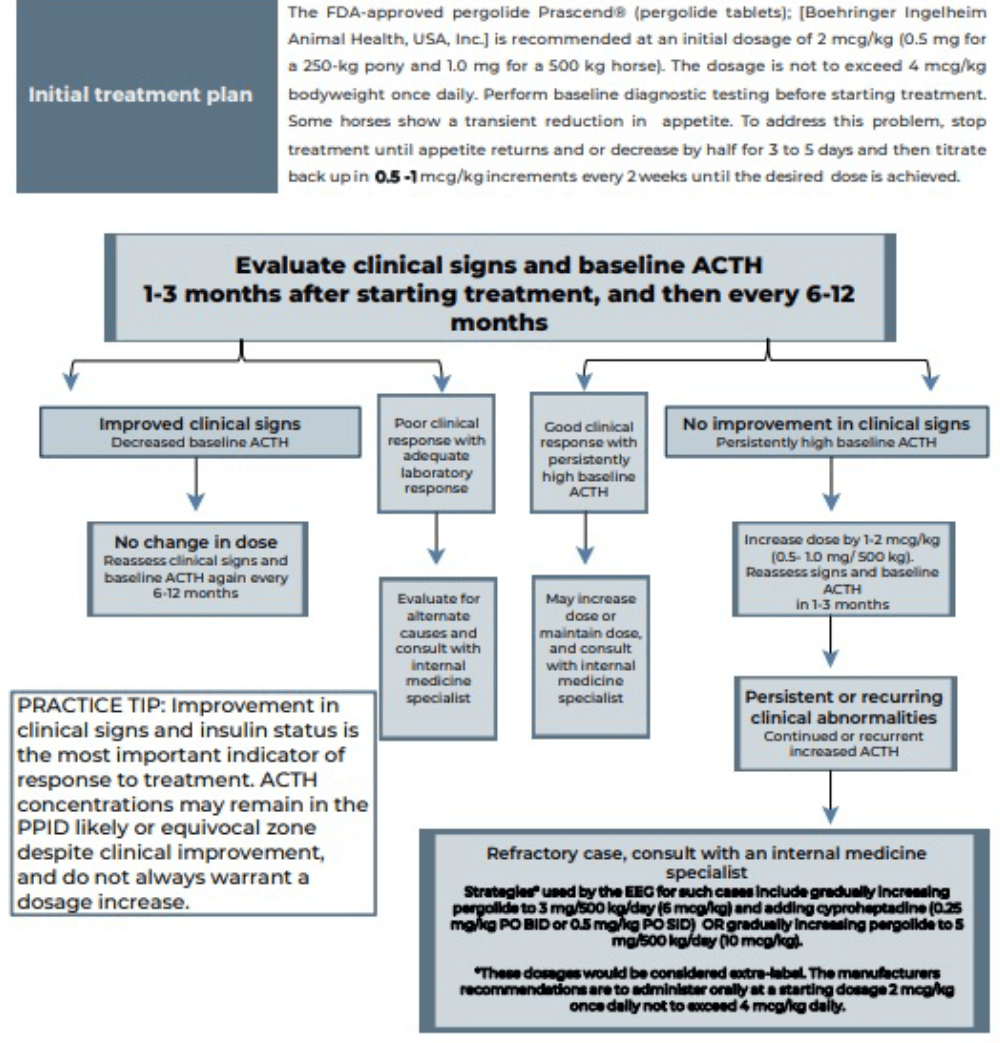
Laminitis is the most common clinical sign that will enforce the use of pharmacological agents to help control the disease. Pergolide is a dopamine agonist that is available as a product licensed in the UK for the treatment of PPID in horses. It is reported to be effective in 65% to 80% of cases. Side effects include anorexia, diarrhoea, depression and colic; however, only anorexia and depression are reported with any frequency. See Figure 4 for details.
Managing acute laminitis
Treatment of the acute laminitis involves providing analgesia and foot support, as well as treating the underlying endocrinopathy.
Analgesia
NSAIDs are the first choice for analgesia and no evidence exists to suggest that any one specific NSAID is superior to the next. If NSAIDs do not provide sufficient pain relief then opiates can be used in addition – for example, butorphanol, pethidine and morphine (used under the cascade). Transdermal fentanyl and oral tramadol (both used under the cascade) can also be considered.
If single drugs do not provide adequate analgesia then multimodal therapy can be used in the hospital setting. Possible combinations typically involve NSAID administration in combination with a constant rate infusion of lidocaine, ketamine, butorphanol, α2 agonists or combinations thereof.
A neuropathic component to the pain associated with laminitis has been demonstrated, making ketamine and gabapentin (used under the cascade) potentially suitable drugs. Newer therapies, such as soluble epoxide hydrolase inhibitors and vanilloid receptor antagonists, may prove useful in the future, but again further work is needed.
Foot support
Supporting the foot is essential. Additional support should be applied to the caudal two-thirds of the foot to provide pain relief and to minimise the mechanical forces on the laminae and, hence, pedal bone movement. The simplest method is to increase the depth of the bedding, ensuring that the bedding extends to the door where the horse will spend most of its day.
Alternatively, or additionally, extra support can be applied directly to the foot itself using methods that can be broadly divided into frog only supports and combined frog and sole supports. No evidence exists to suggest that any one foot support method is superior.
Vasodilator therapy
Vasodilator therapy was historically used based on laminitis being a consequence of digital hypoperfusion. However, this pathogenesis concept is now outdated for endocrinopathic laminitis.
Nevertheless, the sedative effect of acepromazine may have the additional beneficial effect of reducing movement or even resulting in increased periods of time spent recumbent with the weight taken off the feet.
Cryotherapy
Currently, only one published study exists that reports a beneficial effect of digital cryotherapy when initiated at the same time as experimental induction of endocrinopathic laminitis. However, further research is required to determine whether it has a beneficial effect for either the treatment or prevention of endocrinopathic laminitis.
Further reading
Useful resources for the diagnosis and treatment of EMS and PPID can be found on the Equine Endocrinology Group website.
