2 May 2023
Nicola Menzies-Gow looks at this painful but common condition in equines, and explores the links with hyperinsulinaemia and pituitary pars intermedia dysfunction.

Image © skumer / Adobe Stock
Endocrinopathic laminitis is the most common form of the clinical syndrome and encompasses laminitis associated with insulin dysregulation (ID). ID is defined as abnormal insulin metabolism in response to a normal physiologic process, such as eating. In horses and ponies, it manifests in three forms: basal hyperinsulinaemia, in which circulating insulin concentrations are consistently high; an excessive insulin response to carbohydrate, in which ingestion of sugar or starch results in abnormally high circulating insulin concentration peaks; and tissue insulin resistance, in which the resistance is at the level of the tissues. Any combination of these three forms can exist in an individual animal.
It is well established that hyperinsulinaemia induces laminitis in horses and ponies; however, the exact mechanism remains to be determined. The mechanism underlying ID in equine metabolic syndrome (EMS) is also unclear, but evidence exists for a role for genetics, epigenetics, the microbiome, obesity, diet, and endocrine-disrupting chemicals. Similarly, the mechanism by which pituitary pars intermedia dysfunction (PPID) is associated with ID has yet to be determined; however, it is postulated that some of the pars intermedia hormones antagonise insulin.
Alternatively, in some cases, the animal has both EMS and PPID, and so the ID preceded the PPID, and might not have been recognised. Management of endocrinopathic laminitis should focus on identifying and treating the underlying endocrinopathy, and providing appropriate analgesia and foot support.
Laminitis is a clinical syndrome associated with systemic disease (sepsis or systemic inflammatory response syndrome [SIRS] or endocrine disease), or altered weight bearing, rather than being a discrete disease entity1.
Three forms of laminitis exist:

ID is defined as abnormal insulin metabolism in response to a normal physiologic process, such as eating. In horses and ponies, it manifests in three forms: basal hyperinsulinaemia, in which circulating insulin concentrations are consistently high; an excessive insulin response to carbohydrate, in which ingestion of sugar or starch results in abnormally high circulating insulin concentration peaks; and tissue (peripheral) insulin resistance, in which the resistance is at the level of the tissues. Any combination of these three forms can exist in an individual animal.
It is well established that a link exists between hyperinsulinaemia and laminitis in horses and ponies.
Field-based studies have shown an association between hyperinsulinaemia, or other components of ID, and laminitis3,4, and experimental studies demonstrated laminitis can be induced by 48 to 72 hours of insulin infusion in ponies5 and horses6. However, exactly how hyperinsulinaemia causes laminitis remains to be determined.
Theories including glucose deprivation, glucotoxicity, matrix metalloproteinase upregulation and increased blood flow-induced hyperthermia have been disproved.
Current theories include endothelial dysfunction resulting in vasoconstriction, inappropriate signalling via binding of insulin to insulin-like growth factor receptors, and gut microbiota and gut permeability changes, although none of these have yet to be definitively proven.
The exact mechanism underlying ID in EMS remains to be determined. Evidence exists for a role for:
Horses with EMS are often considered to be “good-doers” or “easy keepers”, and genes contributing to this phenotype are likely to be advantageous for survival in the wild during periods of famine by enhancing feed efficiency.
Initial research found the prevalence of laminitis was consistent with the action of a major gene or genes expressed dominantly, but with reduced penetrance attributable to sex-mediated factors, age of onset and further epigenetic factors7. More recently, genome-wide association studies using Arabian horses8, Welsh ponies9 and Morgan10 horses have identified several candidate genes that were associated with relevant traits including height and insulin, triglyceride and adiponectin concentrations. This is an area of ongoing research.
The environment in utero will influence the foal later in life. Certain genes will get switched on or off depending on the nutritional status of the mother, which then influence the offspring later in life.
In people, both maternal overnutrition and undernutrition appear to increase the risk of obesity, and of being metabolically unhealthy when the children reach adulthood. Research in horses is limited, but foals born to overweight mares had ID, chronic low-grade inflammation and an increased risk of developing osteochondrosis at 12 months of age compared to those born from normal-weight mares11.
The gastrointestinal microbiome influences metabolism and endocrine signalling in humans. For example, perturbations of the faecal microbiome in humans and mice have been associated with alterations in insulin secretion and sensitivity, incretin (gut-derived hormones) action and obesity – that is, a microbe-gut-endocrine axis.
Few equine studies exist, but horses with EMS have been shown to have a decreased gastrointestinal microbial diversity and differences in community structure compared to insulin sensitivity control animals12.
Adipose tissue is the largest endocrine organ in the body, producing an array of hormones (adipokines), with normal physiological roles.
Obesity results in adipose dysregulation, such that the production of some adipokines is increased (for example, leptin), and others decreased (for example, adiponectin).
Some of the affected hormones increase or decrease insulin sensitivity. In horses, obesity is associated with increased circulating leptin concentrations, a hormone that amplifies the process of ID in other species.
Diets high in non-structural carbohydrate will reduce insulin sensitivity in horses compared to forage or fat-rich diets13.
Endocrine-disrupting chemicals (EDCs) are found in numerous commercially produced compounds, including organochlorine pesticides such as dichlorodiphenyltrichloroethane, and as by-products during synthesis of various chlorophenols and herbicides. They tend to be polychlorinated, lipophilic and persist in the environment.
Exposure to EDCs is associated with metabolic syndrome, obesity and type-two diabetes in humans. EDCs have been shown to be present in horse plasma and animals living within a short radius of superfunds (chemical dump sites in the US) appear to be at an increased risk of EMS14.
We know only a subset (approximately 25%) of animals with PPID have ID and that the risk of laminitis is greatest in this subset15.
The mechanism by which PPID causes ID has yet to be determined; however, it is postulated some of the pars intermedia-derived hormones antagonise insulin – for example, b-cell tropin, which is a breakdown product of corticotropin-like intermediate peptide.
Alternatively, in some cases, the animal has both EMS and PPID, and so the ID might have preceded the PPID, and just not been recognised.
ID is the central feature of EMS and the subset of animals with PPID that develop laminitis appear to have ID; therefore, investigation of endocrinopathic causes of laminitis requires identification of ID in suspect EMS and PPID cases, and detection of PPID in suspect PPID cases.
Altered circulating concentrations of adipose-tissue-derived hormones (adipokines), such as adiponectin, are found in a proportion of animals with EMS, and so can provide additional information.
ID manifests in three forms and any combination of these manifestations may occur in an individual animal. Therefore, it is important to test for all three (Figure 1).
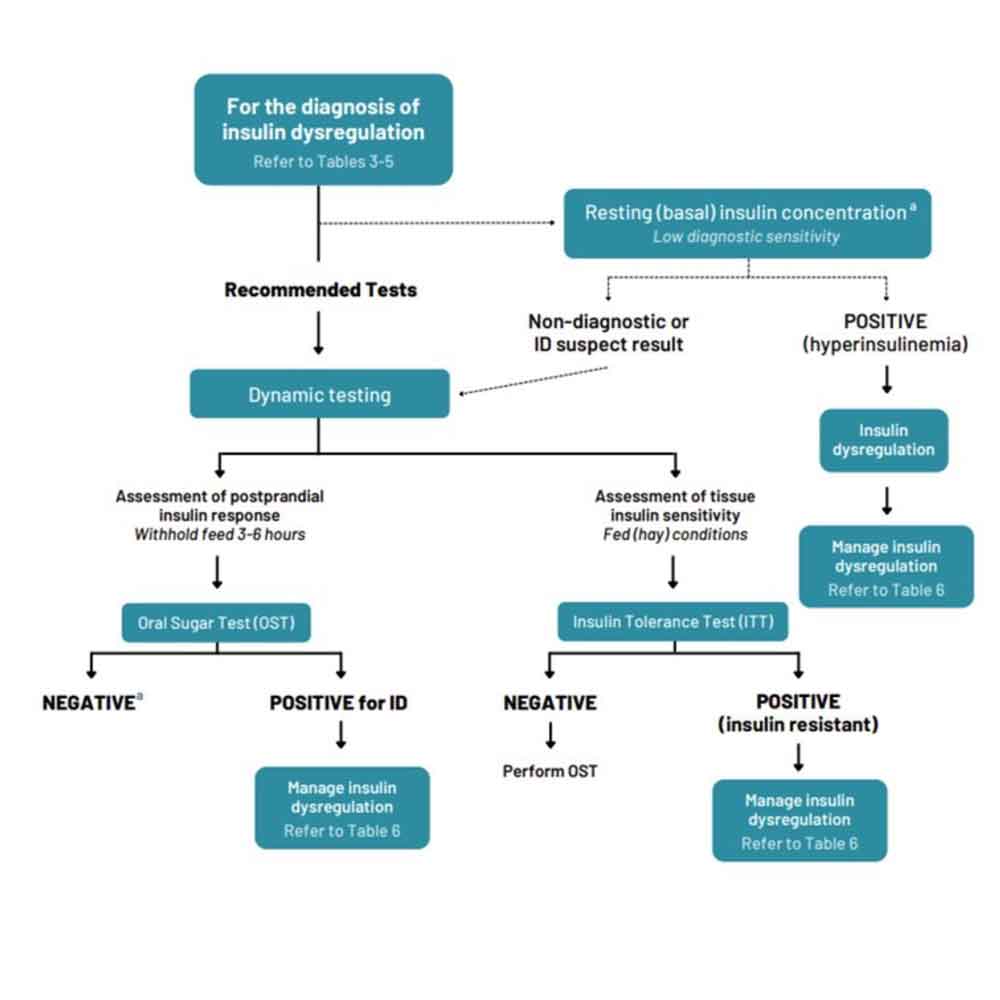
Hyperinsulinaemia is detected by measuring basal insulin concentrations in a single blood sample collected with the horse in the fed state (hay or pasture, but not grain). See Table 1 for interpretation guidance.
| Table 1. Resting insulin concentration interpretation (Equine Endocrinology Group, 2022) | ||
|---|---|---|
| Uses: only used for convenience sampling or monitoring. Will only identify more severely affected animals (test has low sensitivity/high specificity) Update: evidence is mounting that insulin concentrations are affected by season, with higher concentrations detected in December, January and February in the Northern Hemisphere, suggesting winter-associated exacerbation of insulin dysregulation (ID) |
||
| Procedure | After hay (no grain). Do not feed within four hours. Collect into serum or EDTA tube (check with laboratory; assessment of the horse) | While on pasture. Used to assess insulin concentrations during grazinga (assessment of current management) |
| Assays used | Results must be interpreted in the contrast of the insulin assay used (chemiluminescent assay, radioimmunoassay or ELISA). | |
| Results | Interpretationb | Recommendation |
| Radioimmunoassay (RIA) and Immulite 1000: <20µU/mL Immulite 2000 XPi: <31µU/mL |
Non-diagnostic | Dynamic test recommended to better assess |
| RIA and Immulite 1000: 20µU/mL to 50µU/mLc Immulite 2000 XPi: <31µU/mL |
ID suspect if consistent clinical signs | Dynamic test recommended to better assess |
| RIA and Immulite 1000: >50µU/mLc Immulite 2000 XPi: >75µU/mL |
ID | Proceed with ID management |
| *Resting insulin concentrations are not ideal for diagnosis of ID because they are significantly dependent on diet and should be interpreted accordingly. | ||
| a. Values reflect the non-structural carbohydrates (NSC) content of the grass consumed at the time of testing. | ||
| b. Quality and NSC content of forages can vary, and affect results; cut-off values for horses on low-NSC hay. | ||
| c. Note that an insulin concentration of 47µU/mL is used to define a positive response for the oral sugar test (OST), compared to 50µU/mL for resting insulin concentrations. This discrepancy exists because the OST is performed after fasting, whereas resting insulin concentrations are measured in the fed state. | ||
An oral sugar test (OST) is a dynamic test recommended to detect an excessive insulin response to carbohydrate. The OST is preferred because the insulin concentrations measured reflect a more complete sequence of events, including digestion and absorption of sugars, incretin hormone responses and secretion of insulin from the pancreas.
Advantages of this test include the ready availability of commercial corn syrup, ease of administering corn syrup, and the test’s assessment of insulin responses to ingested sugars. Disadvantages include the requirement recommendation for horses to ideally be fasted for three to six hours prior to testing; however, fasting conditions are often achieved by the owner leaving one flake/slice of hay with the horse before midnight and the test is performed the following morning. Alternatively, the test can be performed while the horse remains at pasture.
In situations where horses are resistant to oral administration, the corn syrup can be mixed with a small amount of low-glycaemic feed (for example, chaff). Oral glucose dextrose powder can alternatively be used and should also be mixed with a small amount of low-glycaemic feed. See Table 2 for test details and interpretation guidance.
| Table 2. Interpretation of dynamic testing results (Equine Endocrinology Group, 2022). | ||
|---|---|---|
| Postprandial insulin response | Insulin sensitivity | |
| Oral sugar test (OST)a | Insulin tolerance test (ITT)b | |
| Procedure | Fast three to six hours. Administer 0.15mL/kg or 0.45mL/kg corn syrup orally via dose syringe. Collect blood at 60 and/or 90 minutes. Measure insulin and glucose. |
Fed (pasture or hay) state; do not fast. Collect blood at time zero and administer 0.10IU/kg regular (soluble) insulin. Collect blood at 30 minutes. Measure glucose. Feed hay and small amount of grain immediately after last sample. It is not recommended to perform the OST and ITT on the same day. |
| Interpretationc | >45µU/mL positive for 0.15mL/kg test and radioimmunoassay (RIA). >65µU/mL for 0.45ml/kg test and RIAd >63 µU/mL positive for 0.45mL/kg test and Immulite 2000 XPi. Assess baseline (fasting) glucose concentration to detect diabetes mellitus (rare). |
<50% decrease in blood glucose concentrations from baseline is consistent with insulin resistance. |
| Alternative tests | In-feed oral glucose tolerance test. | Combined glucose-insulin test. |
| a. Use a higher dose of corn syrup (0.45mL/kg) improves test performance5. | ||
| b. Note hypoglycaemia is a risk associated with this test. | ||
| c. Try to minimise stress prior to testing. | ||
| d. Based upon RIA used by the Cornell University laboratory and an initial study with 14 ponies (Menzies-Gow, unpublished data). | ||
The insulin tolerance test (ITT) is recommended to detect peripheral (tissue) insulin resistance.
Advantages of the ITT are that it does not require pre-test fasting, and blood glucose concentrations can be measured with a glucometer so preliminary results are available on the farm. Disadvantages include the cost of purchasing the insulin and the very small risk of clinical hypoglycaemia developing. See Table 2 for test details and interpretation guidance.
Measurement of basal adrenocorticotropic hormone (ACTH) concentration is a practical test for diagnosis of PPID, although variation in individuals and over time results in some overlap in ACTH concentrations between normal and PPID populations. As ACTH concentrations increase in the autumn in healthy and PPID animals, interpretation of ACTH testing for PPID diagnosis requires seasonally adjusted values.
When basal ACTH concentrations are in the equivocal zone, veterinarians should use clinical information such as age and severity of signs to decide whether to monitor and re-test, follow up with dynamic thyrotropin-releasing hormone (TRH) stimulation testing or, in some cases, to treat.
Because the basal ACTH concentration is more likely to fall in the equivocal zone in early-stage disease, the TRH stimulation test may also be an appropriate first-line test in many cases. See Figure 2 and Table 3 for test interpretation guidance.
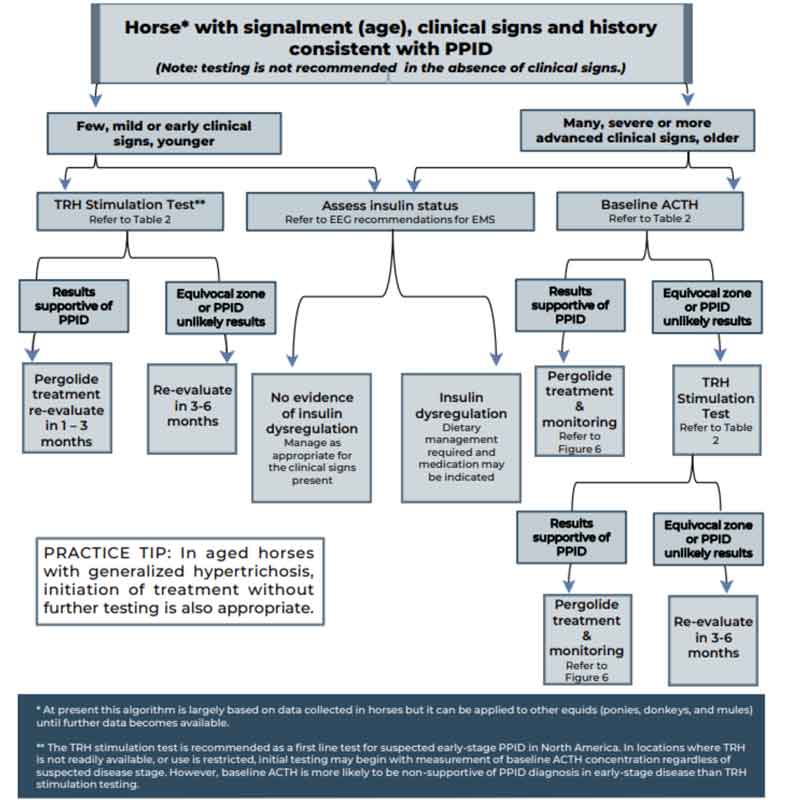
| Table 3. Interpretation of baseline adrenocorticotropic hormone (ACTH) concentration and thyrotropin-releasing hormone (TRH) stimulation test (Equine Endocrinology Group, 2021) | |||
|---|---|---|---|
| Seasonal interpretation of results* | Pituitary pars intermedia dysfunction (PPID) unlikely | Equivocal* (requires strong clinical signs, re-testing or TRH stimulation to confirm diagnosis) | PPID likely |
| Baseline ACTH or TRH time 0 (pg/ml) *see important note below | |||
| December-January | <15* | 15-40* | >40 |
| July and November | <15* | 15-50* | >50 |
| August | <20* | 20-75* | >75* |
| September to October | <30* | 30-90* | >90 |
| 10 minutes after TRH (pg/ml)/strong> | |||
| January to June | <100 | 100-200 | >200 |
| July to December | <100 | TRH stimulation testing can only be used to identify negative cases in these months due to many false positives. | |
| Important notes: The analyser used to measure ACTH has changed since the 2019 EEG recommendations were released, with the Immulite 2000 XPi chemiluminescent immunoassay now used in many laboratories. ACTH values are lower with this method, so ACTH concentrations provided here have been adjusted accordingly. In addition, the equivocal zone for baseline ACTH has been expanded to maximise diagnostic accuracy outside of this zone, and to emphasise the importance of assessing results in the context of the horse’s clinical signs. Current data in a large group of animals with suspected PPID suggests approximately 25% of horses and ponies with PPID, particularly those of certain breeds, may have results that are in this equivocal zone; a diagnosis of PPID is still appropriate in an aged animal when strong clinical signs are present. Up to 30% of horses may have results that fall within the equivocal zone because of stress, breed, and other factors, and are likely to have PPID if strong clinical signs are not present. Further study to refine these criteria in animals with and without confirmed PPID is needed. Re-evaluation of baseline ACTH concentration in 3-6 months or follow-up TRH stimulation testing is recommended for most animals with results in the equivocal zone. A board-certified internal medicine specialist should be consulted for complicated cases. |
|||
Concentrations of the adipokine adiponectin are decreased in a proportion of animals with EMS and associated with an increased risk of future endocrinopathic laminitis independent of insulin concentration16. Measurement provides additional information relating to laminitis risk.
Management of a case of endocrinopathic laminitis involves managing the underlying endocrinopathy, as well as the laminitis itself.
Management of ID consists of dietary modification, exercise and pharmacologic agents.
Dietary modification recommendations depend on whether the animal is obese or lean.
Obesity is managed primarily via energy restriction through limiting intake. An ideal target for weight loss is 0.5% to 1% of body mass (BM) weekly. A daily allowance of 1.25% to 1.5% of actual BM as dry matter intake (DMI), or 1.4% to 1.7%t of actual BM as fed is widely recommended.
In horses with weight loss resistance, a further forage restriction to 1% BM as DMI or 1.15% BM as fed may be considered if appropriately monitored. Grains or cereal-based feeds should be excluded due to their high content of non-structural carbohydrate (NSC), and high-fat feeds should be avoided due to their high energy content. Additionally, large amounts of fruit or vegetables, such as carrots, apples or treats with a high glycaemic load, should be avoided.
The nutrient composition of the forage should be determined where possible and hays with low NSC content (less than 10%) are recommended to limit postprandial insulin responses. Soaking for one hour in warm water or six hours in cold water is advised to reduce the NSC content of the hay if necessary.
Straw is a cost effective and low-energy forage that may be used as an alternative to hay alone. As forages can be low in protein, and mineral and vitamin-leaching occurs after soaking, these nutrients must be balanced by low calorie supplements to cover requirements.
During the initial 6 to 12 weeks of dietary restriction, pasture access should be prevented, as even partial access is very difficult to quantify.
However, successful long-term management of EMS cases can still include some grazing, provided that the ID – especially assessed by the insulin response to oral carbohydrates or grazing – is under control and that grazing is also carefully controlled.
The use of dietary supplements such as cinnamon, magnesium and chromium to facilitate weight loss or to improve ID is popular, but their efficacy remains questionable or unproven.
Lean animals should be fed a low glycaemic diet to minimise the postprandial insulin response.
The diet should be based on forage with a low (ideally less than 10%) NSC content and additional calories provided in the form of fat (for example, vegetable oil), and high-quality fibre such as beet pulp. A low-calorie vitamin, mineral and protein ration balancer should be fed as required.
Exercise has been shown to improve insulin sensitivity in people; however, studies in horses have shown variable results. Exercise should only be considered in animals without current laminitis and should be gradually increased based on fitness.
The optimal exercise regime has yet to be determined; however, half-hour sessions of exercise that raise heart rate to more than 150 beats per minute, performed five days per week, are recommended.
Metformin is the most common drug that has been prescribed for managing ID in horses. Doses range from 15mg/kg to 30mg/kg, given 2 to 3 times daily by mouth, and it is recommended the drug is ideally administered 30 to 60 minutes prior to feeding. However, the oral bioavailability is extremely poor (just 7%), and the drug does not appear to improve insulin sensitivity in equines. However, it had a beneficial effect through blunting of postprandial increases in glucose and insulin concentrations in a single study17.
Levothyroxine (0.1mg/kg to 0.15mg/kg once daily by mouth) may be used in obese animals to accelerate weight loss through increasing the metabolic rate, but must be used in conjunction with diet and exercise changes, as reported side effects include polyphagia. The dose should be gradually reduced and then treatment discontinued after the weight loss has been achieved, or after three to six months of therapy.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors may prove useful, but more scientific evidence is needed. Velagliflozin is a SGLT2 inhibitor that reduces renal glucose reabsorption, promotes glucosuria and, consequently, decreases blood glucose and insulin concentrations in humans. It has also been shown to reduce hyperinsulinaemia and prevent laminitis in insulin-dysregulated ponies fed a challenge diet high in non-structural carbohydrates in a single study with small numbers of animals18; however, it is not commercially available.
Canagliflozin (0.5mg/kg once daily by mouth) was reported to be effective at reducing insulin to normal or near normal concentrations in a US study using 10 horses with hyperinsulinaemia refractory to diet control19, and ertugliflozin (0.05mg/kg once daily by mouth) has been shown to have a similar effect in a study using 51 animals20.
Lipid mobilisation is stimulated in many horses treated with SGLT2 inhibitors and hypertriglyceridemia may develop as a consequence; therefore, triglyceride concentrations should be closely monitored (days zero, seven and 14, and every one to three months thereafter), and the benefit of continued use in individuals with marked hypertriglyceridaemia discussed with the owner.
It should be remembered that none of these drugs are licensed for use in horses and each is used in accordance with the cascade.
PPID is a slowly progressive, lifelong condition. The aim of treatment is not to cure the condition, but to increase the quality of life through reducing the clinical signs, including those that have the potential to be life threatening.
Laminitis is the most common clinical sign that will enforce the use of pharmacological agents to help control the disease. Pergolide is a dopamine agonist that is available as a product licensed in the UK for the treatment of PPID in horses. It is reported to be effective in 65% to 80% of cases.
Side effects include anorexia, diarrhoea, depression and colic; however, only anorexia and depression are reported with any frequency (Figure 3).

If compliance with pergolide tablets is limiting, pergolide is also available as an unlicensed paste formulation. Although a licensed medicine should be the first choice for treating any medical condition, if another product is more suitable for an individual horse, that product can be used if “clinical justification” exists for doing so – for example, if not taking the licensed medicine could lead to welfare issues/a decline in a horse’s clinical condition.
For example, some horses will not eat tablets in any form, or may need dosing increments less than the 0.5mg that tablets can be split into.
The BOVA pergolide paste is dispensed in 0.2mg increments and being molasses-flavoured has been found to be readily accepted by horses in trials21.
Cabergoline is also a dopamine agonist that may be a potential replacement for pergolide for use in horses with PPID under certain circumstances due to its long-acting nature. In one study, a single injection of 5mg cabergoline (in a slow-release vehicle) effectively applied dopaminergic activity to healthy horses for 7 to 10 days22. No licensed product is available for use in the horse, but it is available in an unlicensed injectable formulation.
Treatment involves providing analgesia and foot support, as well as treating the underlying endocrinopathy.
NSAIDs are the first choice for analgesia and no evidence exists to suggest any one specific NSAID is superior to the next. If NSAIDs do not provide sufficient pain relief, then opiates can be used in addition – for example, butorphanol, pethidine and morphine. Transdermal fentanyl and oral tramadol can also be considered.
If single drugs do not provide adequate analgesia, then multimodal therapy can be used in the hospital setting. Possible combinations typically involve NSAID administration in combination with a CRI of lidocaine, ketamine, butorphanol, alpha-2 agonists or combinations thereof. A neuropathic component to the pain associated with laminitis has been demonstrated, making ketamine and gabapentin potentially suitable drugs. Newer therapies, such as soluble epoxide hydrolase inhibitors and vanilloid receptor antagonists, may prove useful in the future, but further work is needed.
Supporting the foot is essential. Additional support should be applied to the caudal two thirds of the foot to provide pain relief and to minimise the mechanical forces on the laminae, and hence pedal bone movement.
The simplest method is to increase the depth of the bedding, ensuring the bedding extends to the door where the horse will spend most of its day. Alternatively, or additionally, extra support can be applied directly to the foot itself using methods that can be broadly divided into frog-only supports and combined frog and sole supports. No evidence exists to suggest that any one foot support method is superior.
Only one published study exists that reports a beneficial effect of digital cryotherapy when initiated at the same time as experimental induction of endocrinopathic laminitis. However, further research is required to determine whether it has a beneficial effect for either the treatment or prevention of endocrinopathic laminitis.