8 Dec 2020
Endoparasite control in adult horses – a modern approach
Zoë Gratwick discusses the diagnosis of tapeworm and strongyle infections, as well as when to use anthelmintic treatments.

Two of the most pertinent questions in equine endoparasite control are “what would be best for the individual at present?” and “what would be best for preserving future anthelmintic efficacy?”.
These are not necessarily opposing in nature, although this is sometimes perceived to be the case.
Little exists to suggest that low parasite burdens cause disease. Mammals have evolved alongside helminth infections, and low-level burdens may, in fact, be beneficial.
Helminths play an immunomodulatory role – and evidence is growing in other species that their presence is important in the development of immune responses, and in the mitigation of autoimmune and allergic diseases1,2.
Importantly, acquired immunity plays a role in host susceptibility to nematode infections. In sheep, frequent anthelmintic exposure leads to a return to susceptibility in previously immune adult animals3.
The implications of different dosing regimens on equine immunity is poorly understood, but initial evidence suggests highly frequent administration of anthelmintics may have an adverse effect4,5.
Anthelmintics are administered to prevent parasitic disease. Therefore, knowing the level of burden at which parasites are likely to cause disease would be useful. Unfortunately, this remains unclear – and for this reason advice consists of sensible suggestions using the information available to us, with the aim of concurrently preventing disease and minimising anthelmintic usage.
It should be expected that recommendations will evolve over coming years as further information becomes available.
This article will focus on the diagnosis and control of tapeworm and strongyle infections. Other endoparasites – such as the ascarid species, Habronema species, Dictyocaulus arnfieldi, Oxyuris equi and Fasciola hepatica – can also cause disease in adult horses and may require consideration in certain circumstances.
The control of parasitic disease in youngstock is beyond the scope of this article, but information on this subject can be found elsewhere6.
Endoparasitic disease in adult horses
Encysted cyathostomins typically inhabit the large intestinal wall, where they may cause tissue damage and subsequent diarrhoea (either acute or chronic), colic symptoms or weight loss (Figure 1)7.

In the UK, cyathostomins are thought to be the most common cause of parasitic disease in adult horses.
The tapeworm Anoplocephala perfoliata most frequently inhabits the ileocaecal junction and caecum (Figure 2)8. It is often considered to be associated with colic, with spasmodic colic cases being 15 times more likely to be infected than control horses and ileal impaction cases being 44 times more likely to be infected9.
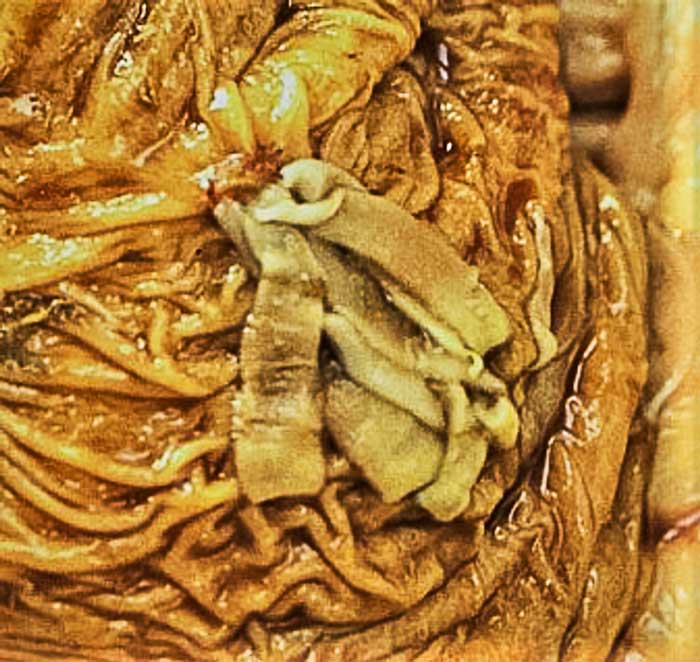
A number of case reports have suggested an association between A perfoliata and ileocaecal, caecocolic and caecocaecal intussusceptions, as well as caecal and ileal ruptures10-12. Although these reports highlight a probable role of A perfoliata in the development of colic cases, they do not prove a causative relationship.
Additionally to their likely ability to affect intestinal motility, A perfoliata can cause inflammation and even necrotic ulceration at sites of attachment13.
Heavy burdens of both small and large adult strongyles can lead to weight loss and poor thrift. Furthermore, migrating Strongylus vulgaris larvae can lead to cranial mesenteric artery occlusion with subsequent intestinal infarction and colic symptoms7. This was once a common disease, reduced significantly by the introduction of widespread effective anthelmintic use. However, concerns exist about its re-emergence as anthelmintic resistance develops.
For the individual, the scenario of a clinical case being untreatable due to anthelmintic resistance is a grave one. Furthermore, the implications for equine businesses, such as stud farms, can be wide-reaching.
The overall administration of less anthelmintic doses is thought to be a key step in reducing the development of resistance in equine parasites. This does not mean withholding indicated anthelmintics, but, rather, avoiding unnecessary usage.
Vital underpinning factors in this approach include environmental management to reduce exposure through contaminated pasture (Panel 1) and the use of targeted deworming strategies.
- Horse numbers per acre should be kept low to prevent overgrazing and to reduce contamination by worm eggs. One horse per acre is an appropriate rule of thumb.
- Pick up and dispose of faeces regularly (at least twice per week). Do not spread this on to fields grazed by horses – compost it away from grazing.
- Consider grazing horses with sheep or cattle as they will reduce the number of infective larvae (of equine parasites) on a pasture.
- Grazing horses that repeatedly have high egg counts separately from other horses can help to reduce infection rates in the rest of the group. These animals are commonly youngsters or geriatric horses.
Endoparasite diagnostics: moving away from blanket treatment
Faecal egg counts
The once common practice of regularly deworming all horses based entirely on the number of days since the previous anthelmintic treatment appears to be reducing.
Faecal egg counts are widely used to gain an approximate idea of the burden level of adult strongyles (large and small). Ascarid and other ova may also be identified, and are typically listed separately and excluded from the main count.
Typically a cut-off of greater than 200epg to 300epg strongyle‑type ova is used to indicate treatment requirement in adult horses. However, in healthy adult horses kept at a low stocking density on well‑managed pastures, a cut-off of greater than 500epg may be appropriate17.
Faecal egg counts alone cannot offer reliable information about encysted cyathostomin and A perfoliata infections. For this reason, in many cases faecal egg counts have been used to assess the need for treatment of adult strongyle infections, but 6-monthly to 12‑monthly treatment for encysted cyathostomin and tapeworm infections has continued.
However, recent diagnostic developments have enabled an improved, even more targeted approach to encysted cyathostomin and A perfoliata treatment.
Cyathostomin serology (small redworm ELISA)
The cyathostomin serology ELISA detects antibodies to selected cyathostomin antigens.
Prior to the launch of this test, it was recommended that larvicidal treatment of all adult horses was given in the autumn. Use of this test can provide information about the level of cyathostomin burden and, therefore, can be used to assist veterinary surgeons in deciding whether to advise larvicidal treatment in any given horse.
It is important to note this is a test for all cyathostomin life stages, not a test specifically for encysted larvae. The test developers have advised that the test be used in cases considered to be at low to moderate risk of a cyathostomin burden and that cases considered to be at high risk receive at least annual larvicidal treatment.
Horses considered to be at low risk include those that usually have faecal egg counts of less than 50epg, live in a closed herd where all animals in the herd consistently have faecal egg counts lower than 200epg, and where good pasture management is practised.
Horses considered to be at moderate risk include those that usually have faecal egg counts of less than 50epg, live in a closed herd where a low proportion of the herd consistently has faecal egg counts greater than 200epg, and where moderate pasture management is practised.
High-risk animals are those with a recent faecal egg count greater than 200epg that live in an establishment with a high herd turnover, where a high proportion of the herd frequently has a faecal egg count greater than 200pg, and where poor paddock management is practised.
The test developers highlighted that false negatives could occur in cases with hypoalbuminaemia. This is of particular importance, as many cases of clinical cyathostominosis demonstrate a degree of hypoalbuminaemia.
Like all antibody ELISAs, this test relies on an animal mounting an antibody response to the pathogen in question. It should, therefore, be used with caution in immunocompromised patients where the likely effect on the magnitude of antibody responses is unquantifiable.
It is recommended that this test is not used within four months of larvicidal treatment, to reduce the likelihood of the detection of persistent antibodies from a previously resolved infection.
Tapeworm serology (serum ELISA) and the tapeworm saliva ELISA
Recent exposure to tapeworms can be determined using serum and saliva tests. Furthermore, these techniques are semi‑quantitative and indicate the intensity of exposure.
An association has been demonstrated between the infection intensity of A perfoliata and the extent of local damage caused; it has been suggested that burdens of more than 20 worms are more likely to cause significant damage than lower burdens18. Both the serum ELISA and the saliva-based ELISA have been demonstrated to correctly identify burdens or more than 20 worms approximately 100% of the time19.
As with nematode infections, a small proportion of horses carry high tapeworm burdens, with a much greater percentage (approximately 78%) persistently falling below a moderate/high threshold20.
It has been recently demonstrated that in one large herd, the use of the saliva-based diagnostic test to judge requirement for tapeworm treatment led to an 86% reduction in the number of treatments that would have been given if a twice‑yearly interval dosing programme had been used without testing20.
Tapeworm burdens are thought to be highest in the autumn; therefore, when testing annually, this would be the best time to do so21.
Exactly how long antibody levels take to normalise following treatment could be affected by a number of factors – in particular how high the antibody level was prior to treatment.
Preliminary results for the saliva-based tests suggest that, in most cases, salivary antibody levels are low within three months of treatment. Serum antibodies are thought to take at least four months to normalise post-treatment.
It is advised that food and water are not consumed within half an hour of the tapeworm test being performed. It is also recommended that horses are not exercised within 30 minutes of testing.
Additionally, it is not recommended that this test be done following recent administration of alpha-2 adrenergic agonist sedatives.
Anthelmintic treatments: what and when?
Encysted cyathostomins
Both moxidectin and fenbendazole are licensed in the UK for the treatment of encysted cyathostomins.
The extent and national distribution of resistance is unknown. However, in a study looking at 17 UK yards, a degree of fenbendazole resistance was apparent at 82% of premises (versus 0% for moxidectin). For this reason, when specifically targeting encysted cyathostomins, moxidectin is typically advisable17.
The notion of using fenbendazole wherever possible, to reserve moxidectin, is appealing. However, no antemortem way exists of determining whether encysted stages are sensitive to fenbendazole.
If required (see aforementioned guidance), moxidectin treatment should ideally be given around October, as this is both around the end of the grazing season and prior to the period of most risk. It was previously recommended that this treatment be withheld until after the first frost; however, it’s now believed infective pasture-dwelling cyathostomin larvae can survive a frost.
Tapeworms
It is recommended that treatment for tapeworms is only given either if indicated by ELISA, or in cases where an ELISA may not be appropriate (for example, a hypoalbuminaemic patient)17. Testing is advised in the autumn and combining this with October cyathostomin testing/treatment is usually most practical.
Both pyrantel and praziquantel are licensed for the control of tapeworms. No practical way exists of monitoring for anthelmintic resistance in equine tapeworms and resistance has not been reported to either drug.
If treating for tapeworms specifically, the use of praziquantel – rather than pyrantel – would prevent inadvertent exposure of nematodes to this drug, potentially helping to preserve their sensitivity. Nematodes are not sensitive to praziquantel and, therefore, not thought be affected by exposure to the drug.
A licensed praziquantel product is no longer available in the UK, but can be obtained by veterinary surgeons from a specials manufacturer.
Adult strongyles
It is recommended that any prophylactic treatment is based on faecal egg counts.
Given the essential nature of the preservation of moxidectin for the treatment of encysted cyathostomins, its use is not routinely advisable for other parasites17. Options include a single dose of fenbendazole, ivermectin or pyrantel. In this instance, a faecal egg count reduction test could be used to establish the efficacy of any treatment given; this may be advisable on an annual basis if treatment is required.
Whether cross‑resistance may develop between ivermectin and moxidectin in equine strongyles is unknown, and evidence is mixed in non-equine nematodes.
When pyrantel is used, tapeworms will be inadvertently exposed. As a significant percentage of tapeworms require double the standard dose of pyrantel, a proportion will be exposed to sub-therapeutic levels.
References
- Maizels RM and Yazdanbakhsh M (2003). Immune regulation by helminth parasites: cellular and molecular mechanisms, Nature Reviews Immunology 3: 733-744.
- Furze RC, Hussell T and Selkirk ME (2006). Amelioration of influenza‑induced pathology in mice by coinfection with Trichinella spiralis, Infection and Immunity 74(3): 1,924-1,932.
- Abbott KA, Taylor M and Stubbings LAA (2012). Sustainable worm control strategies for sheep: a technical manual for veterinary surgeons and advisors (4th edn), http://bit.ly/36LJ4vU
- Monahan CM, Chapman MR, Taylor HW, French DD and Klei TR (1997). Foals raised on pasture with or without daily pyrantel tartrate feed additive: comparison of parasite burdens and host responses following experimental challenge with large and small strongyle larvae, Veterinary Parasitology 73(3‑4): 277-289.
- Baudena MA (2003). Equine immunity to cyathostome infections, LSU Doctoral Dissertations: 3180, http://bit.ly/3n5PKFL
- Reinemeyer CR and Nielsen MK (2017). Control of helminth parasites in juvenile horses, Equine Veterinary Education 29(4): 225-232.
- Nielson MK, Reinemeyer CR and Sellon DC (2014). Nematodes. In Sellon DC and Long MT (eds), Equine Infectious Diseases (2nd edn), Saunders, St Louis: 475-489.
- Williamson RMC, Gasser RB, Middleton D and Beveridge I (1997). The distribution of Anoplocephala perfoliata in the intestine of the horse and associated pathological changes, Veterinary Parasitology 73(3‑4): 225-241.
- Proudman CJ and Edwards GB (1993). Are tapeworms associated with equine colic? A case control study, Equine Veterinary Journal 25(3): 224-226.
- Barclay WP, Phillips TN and Foerner JJ (1982). Intussusception associated with Anoplocephala perfoliata infection in five horses, Journal of the American Veterinary Medical Association 180(7): 752-753.
- Owen RA, Jagger DW and Quan-Taylor R (1989). Caecal intussusceptions in horses and the significance of Anoplocephala perfoliata, Veterinary Record 124(2): 34-37.
- Ryu SH., Bak UB, Kim JG, Yoon HJ, Seo HS, Kim JT, Park JY and Lee CW (2001). Cecal rupture by Anoplocephala perfoliata infection in a Thoroughbred horse in Seoul Race Park, South Korea, Journal of Veterinary Science 2(3): 189-193.
- Pearson GR, Davies LW, White AL and O’Brien JK (1993). Pathological lesions associated with Anoplocephala perfoliata at the ileo‑caecal junction of horses, Veterinary Record 132(8): 179-182.
- Daniels SP and Proudman CJ (2016). Shortened egg reappearance after ivermectin or moxidectin use in horses in the UK, The Veterinary Journal 218: 36-39.
- Molento MB, Antunes J, Bentes RN and Coles GC (2008). Anthelmintic resistant nematodes in Brazilian horses, Veterinary Record 162(12): 384-385.
- Traversa D, von Samson‑Himmelstjerna G, Demeler J, Milillo P, Schürmann S, Barnes H, Otranto D, Perrucci S, Frangipane di Regalbono A, Beraldo P, Boeckh A and Cobb R (2009). Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany, Parasites and Vectors 2: S2.
- Rendle D, Austin C, Bowen M, Cameron I, Furtado T, Hodgkinson J, McGorum B and Matthews J (2019). Equine de-worming: a consensus on current best practice, UK-Vet Equine 3(Suppl 1): 1-14.
- Fogarty U, del Piero F, Purnell RE and Mosurski KR (1994). Incidence of Anoplocephala perfoliata in horses examined at an Irish abattoir, Veterinary Record 134(20): 515-518.
- Lightbody KL, Davis PJ and Austin CJ (2016). Validation of a novel saliva-based ELISA test for diagnosing tapeworm burden in horses, Veterinary Clinical Pathology 45(2): 335-346.
- Lightbody KL, Matthews JB, Kemp-Symonds JG, Lambert PA and Austin CJ (2018). Use of a saliva‑based diagnostic test to identify tapeworm infection in horses in the UK, Equine Veterinary Journal 50(2): 213-219.
- Tomczuk K, Kostro K, Grzybek M, Szczepaniak K, Studzińska M, Demkowska-Kutrzepa M and Roczeń-Karczmarz M (2015). Seasonal changes of diagnostic potential in the detection of Anoplocephala perfoliata equine infections in the climate of Central Europe, Parasitology Research 114: 767-772.
