15 Jan 2021
Janet Littlewood discusses the second most common allergic skin disease in horses, which can present a diagnostic and therapeutic challenge.

Although not as common as Culicoides hypersensitivity (CHS; “sweet itch”), atopic dermatitis is not an uncommon presentation in horses, and is the most frequent reason for referral to the author.
The true prevalence is unknown, but atopy accounted for 3.9% of all equine cases seen by the dermatology service at Cornell University College of Veterinary Medicine across a 21-year period. Although a further 4.5% had final diagnoses of idiopathic pruritus or idiopathic urticaria, these cases were most likely atopic. However, further investigations were not undertaken.
Equine atopic dermatitis (EAD) has constituted 36% of the author’s equine referral caseload in the past 13 years. The condition presents a diagnostic and management challenge for veterinarians and owners, particularly as little evidence-based information published remains about diagnosis, management and outcome of cases.
The presenting signs in cases of EAD vary from those seen in canine atopic disease (CAD), with pruritus and/or urticaria being the predominant signs (Table 1). It is not clear why some animals present with exclusively or predominantly one sign or the other. It has no gender predisposition and the condition has been reported in many breeds of equids, with some publications considering certain breeds to be at risk, but usually without any comparison to a reference or standard populations. Familial incidence is recognised in some reports.
| Table 1. History and presenting signs in equine atopic dermatitis (Loeffler et al, 2018) | |
|---|---|
| Presenting sign | Result |
| Age at referral: years, mean (range) | 9.9 (1-25) |
| Pruritus alone | 43.9% |
| Urticaria alone | 41.5% |
| Pruritus and urticaria | 14.6% |
| Generalised distribution | 81.7% |
| Localised (trunk, head, limbs) | 9.7%, 7.3%, 1.2% |
| Affected during winter months | 100% |
| Spring/summer exacerbations | 23.2% |
The age of onset of disease and at presentation is much wider than that seen in CAD, but it should be recognised many horses change ownership, and are likely to be sold in their non-allergic season, so the age of first clinical signs reported by owners may not be a true picture. All of the cases seen by the author have been affected during the autumn/winter months, when the majority of pleasure horses are stabled for most of the time. Some are perennially affected or show spring/summer exacerbations.
Just as flea allergy dermatitis can co-exist with atopic dermatitis in dogs, atopic horses may also suffer from CHS. Distribution of lesions in EAD is usually generalised, but a minority of cases may present with more localised signs. Some cases with EAD may also have concurrent respiratory hypersensitivity disorders, although this is reported in only a minority of the author’s caseload.
The diagnosis of atopic dermatitis is made by a process of elimination of other causes of pruritus and urticaria (Table 2).
| Table 2. Main differential diagnoses to be considered for cases with pruritus and/or urticaria | |
|---|---|
| Pruritus | Urticaria |
| Parasites • Chorioptic mange • Pediculosis • Trombiculidiasis • Poultry mite infestation • Oxyuriasis • Strongyloidosis |
Drug reactions • Topicals • Antibiotics • NSAIDs • Narcotics • Vaccines • Antisera |
| Hypersensitivity disorders • Culicoides hypersensitivity • Other insect-bite hypersensitivities • Atopic dermatitis • Forage mite infestation |
Hypersensitivities • Insects • Atopy • Contact reactions • Drugs |
| Adverse food reactions | Adverse food reactions |
| Contact dermatitis/hypersensitivity | Associated with infections and infestations • Bacterial • Fungal • Viral • Protozoal • Parasitic, including endoparasites • Straw and forage mites |
| Malassezia dermatitis | Arthropod stings or bites |
| Pemphigus foliaceus | Vasculitis |
| Dermatographism | |
| Exercise/heat/cold-induced | |
The importance of taking a good history cannot be overemphasised, followed by thorough clinical examination and some basic diagnostic tests to rule out other causes of pruritus/urticaria (Table 3).
| Table 3. History and presenting signs in equine atopic dermatitis (Loeffler et al, 2018) | |
|---|---|
| Microscopic examinations | • Hair plucks • Superficial material collected by tape strips, combings or scrapings • Cytological examinations of surface adhesive tape strip samples or wet crust preparations |
| Restriction/provocation testing | • Change bedding • Turnout full time • Elimination diet |
As many cases of urticaria in the horse – probably around 60% – are one-off episodes that respond to appropriate symptomatic treatment, further investigation is suggested for cases suffering more than three recurrences or persist for more than six weeks.
Adverse food reactions (AFRs), although commonly suspected in the horse, are rare – as can be evidenced from the lack of a good series of case reports in peer-reviewed literature. Serological tests that purport to aid in the diagnosis of cases of food sensitivity have been shown to be of limited or no validity in small animals, and cannot be recommended in horses where lack of understanding of the mechanisms of disease and lack of positive control reagents to validate assays exists. The only valid way of ruling out AFR is by undertaking an elimination diet to consist of one simple foodstuff alone for a period of a minimum of one month, followed by dietary provocative challenge.
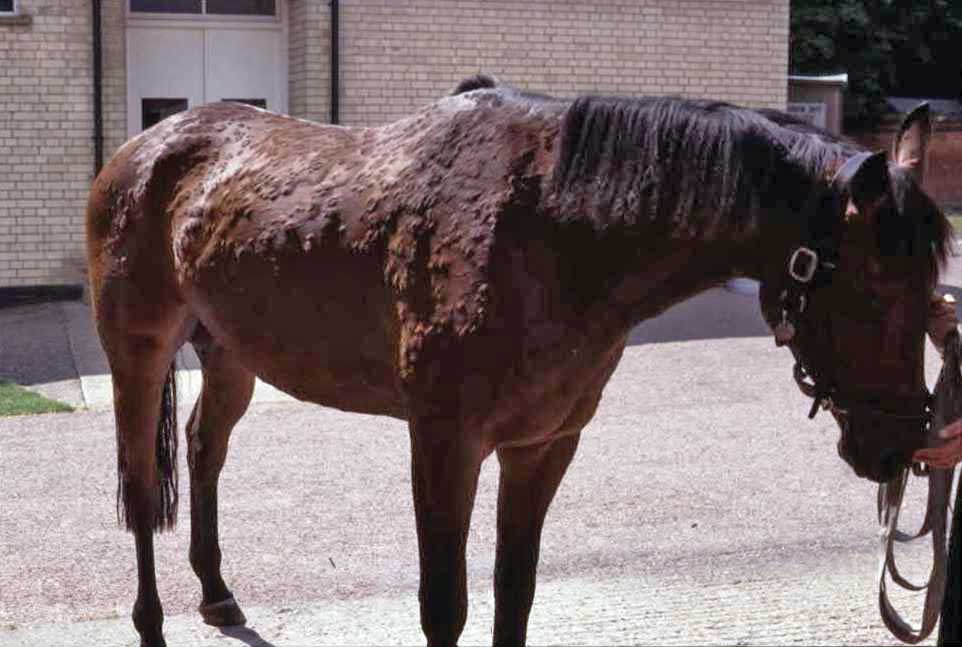
When a clinical diagnosis of atopic dermatitis has been reached, allergen-specific IgE testing should be considered. These tests are not for the diagnosis of allergy, but to aid in the management of cases once other differentials have been excluded. Intradermal testing (IDT) is considered the gold-standard test by dermatologists. However, no universally accepted standards for allergen dilutions and methodology exist, including volume of allergens injected, time points for reading reactions, criteria for measuring and recording wheal sizes of controls, and test injections.

The most common methodology involves sedation – but not with promazine-related drugs – clipping of the test site on the lateral neck or shoulder and examination at 30 minutes (and a later time point of two to four hours to capture late-immediate type 1 hypersensitivity reactions) and ideally further recording 24 to 48 hours later to document delayed, type 4, hypersensitivity reactions. Most dermatologists consider wheal sizes greater than the mean of the positive and negative controls to be positive and indicative of type 1 hypersensitivity.
It is known that normal horses with no evidence of skin disease can show positive skin test reactions, although these are predominantly to biting insects and so likely to represent a normal immunological reaction to a parasite.
Other reasons for false-positive IDT reactions may include irritant allergens, contaminated allergen solutions, poor technique, cross-reactions with ectoparasite antigens, causes of non-immunological histamine release, such as narcotics, and dermatographism. Dermatographism is the mechanical degranulation of mast cells with resultant histamine release, and may be a cause of multiple positive reactions in skin tests, including to the negative control. However, the presence of dermatographism in a case does not rule out the diagnosis of atopic dermatitis as some, though not all, reviewers of urticaria in humans consider that the condition is associated with increased numbers of dermal mast cells usually due to underlying atopy.
False-negative IDT results can be seen due to recent or current treatment with glucocorticoids (both systemic and topical) antihistamines, progestagens and some tranquillisers – all of which can inhibit skin test reactivity. Testing long after the allergy season may give false-negative results due to low levels of tissue-bound IgE; likewise, testing at the height of the allergy season may give false-negative results due to anergy. Other reasons for false-negative IDT reactions include testing with allergen mixes that contain inadequate concentrations of individual allergens, out-of-date allergens, degradation or breakdown of protease allergens, over-dilute solutions or too small a volume of injection.
In summary, the technique of IDT is not without challenges in performance and interpretation, and so should be reserved for use by clinicians with appropriate training and seeing a sufficient caseload to have developed experience in the use of the test, which usually means in a referral setting. Conclusions regarding implicated allergens must take into account the clinical history and likely exposure to decide on the relevance of positive reactions.
Serological tests for the identification of allergen-specific IgE antibodies are offered by several commercial laboratories, and would seem to offer an easily performed, and appealing alternative to IDT, avoiding the risks of false-negative reactions due to drug treatments. However, it has been shown in the dog that steroids can reduce serum IgE concentrations to below the threshold of sensitivity of ELISA assays. Also, and importantly, published data shows the correlation between different serological assays and IDT horses is poor to non-existent.
The least-poor correlation was with use of the Heska Allercept reagent, which uses a recombinant peptide derived from the high-affinity IgE receptor as the capture reagent. A study comparing this reagent and IDT in the dog showed good correlation for the most commonly implicated allergens in CAD – dust mite allergens – but false-negatives were seen in the ELISA compared to IDT for these allergens. Reasons for false-negative ELISA results in comparison to IDT include the lack of correlation between skin-bound and circulating IgE, and the half-life of the plasma IgE being significantly shorter than that bound to mast cells.
False-positive ELISA results may occur due to cross-reactive carbohydrate determinants commonly found in pollens. Reagents in current use in equine assays include polyclonal anti-equine IgE antisera, monoclonal anti-equine IgE antibodies, the high-affinity IgE receptor peptide and a monoclonal antibody generated from recombinant equine IgE. The latter is a more recently developed reagent not available when the published comparison of serological assays and IDT was undertaken (Lorch et al, 2001). This newer reagent is used in assays claimed to be highly specific and highly sensitive for the detection of circulating IgE antibodies in horses (Next Equine, Artuvet; Alergovet Equine ELISA, NWL).
Identification of implicated allergens enables the use of allergen-specific immunotherapy (ASIT) as a treatment option. While the mechanisms of action are not fully understood, ASIT offers the possibility of inducing tolerance to the causal allergens, therefore addressing the underlying immunological problem. Injectable ASIT has been available and in use for many years. The generally held view that ASIT is helpful in about two-thirds of treated animal cases has been validated in the publication by Loeffler et al (2018), which documented benefit in 64.3% of treated horses.
One of the challenges with ASIT is managing the expectations of owners, as a considerable time lag of months can take place before benefit can be expected – a minimum of 9 to 12 months of treatment should be completed before a decision on whether the therapy has been unsuccessful. Because of the delay to onset of benefit, it is vital symptomatic therapy is continued – particularly in the case of pruritic animals, as the welfare significance of pruritus should not be underestimated. Concurrent use of glucocorticoid therapy does not affect the outcome of ASIT. Side effects from injectable ASIT seem to be uncommon, although occasional injection-site reactions are reported and the potential risk of anaphylaxis should be borne in mind.
Although owners can be instructed on administration of monthly maintenance injections, the author advises induction injections be given by veterinarians with observation for at least 15 to 20 minutes afterwards. If reactions are going to occur, it is likely to be after the third, fourth or fifth injections (six induction doses for Artuvetrin therapy are used by the author).
Sublingual immunotherapy has become available for veterinary patients. Although slightly more expensive to purchase initially, this offers an easier option for some owners – particularly for needle-shy horses. It requires daily administration of allergen solution, with split dosing advised by some manufacturers, on to the oral mucosa – the lower lip fold is the easiest site of application. Efficacy of oral ASIT in the dog appears to have a similar efficacy to ASIT injections and, while no published data is available at this time regarding equine use, the author has several horses on oral ASIT with good response and withdrawal of other therapy.
As well as allowing formulation of ASIT, knowledge of implicated allergens enables advice to be given regarding allergen-avoidance measures, which the author has found to be of considerable benefit (Littlewood et al, 1998). Since it is “indoor allergens” such as dust mites, forage mites, feathers and, less commonly, moulds that are frequently implicated, measures such as removal of all loose bedding and replacing with padded rubber matting, removal of nests from stables (after hatching and fledging of chicks) and prevention of future access of birds, quarterly pressure cleaning, regular laundering of rugs to remove dust mite allergens, feeding of vacuum-packed wilted grass products and careful storage of concentrate foods to prevent forage mite contamination may all help reduce allergen exposure.
Where weed pollens are implicated, the use of a broad-leafed herbicide can be employed as part of pasture management.
Unsurprisingly, the most effective symptomatic treatment in EAD is glucocorticoids, with around 80% of cases treated prior to referral and more than 90% of cases treated after referral reported as responding in a follow-up study of cases (Loeffler et al, 2018). Adverse events (AEs) were reported in 20% of steroid-treated cases, of which laminitis was the most common (42.8% of AEs). However, in all cases this was either a flare of pre-existing laminitis or associated with other foot pathology. Lethargy, weight gain and behaviour changes were other reported AEs. Topical glucocorticoids were also helpful – in particular, application of hydrocortisone aceponate spray.
A good response to antihistamines was reported in 60.7% of treated cases, with only drowsiness reported occasionally. Antihistamines can be used in combination with steroids to reduce the doses needed to control pruritus. For cases with intractable pruritus, the additional use of tricyclic antidepressant drugs – such as amitriptyline and doxepin, and others – can be helpful (Table 4).
| Table 4. Drugs that may be helpful for systemic symptomatic therapy for equine atopic dermatitis | |
|---|---|
| Clucocorticoids | • Prednisolone 0.15mg/kg to 1.5mg/kg po sid induction; maintenance 0.2mg/kg to 0.5mg/kg po every other day • Dexamethasone 0.02mg/kg to 0.1mg/kg po or injection loading maintenance 0.01mg/kg to 0.02mg/kg po every two to three days |
| Antihistamines* | • Hydroxyzine 1mg/kg to 2mg/kg bid or tid • Chlorphenamine 0.25mg/kg to 0.5mg/kg bid • Diphenhydramine 1mg/kg to 2mg/kg bid or tid • Alimemazine (trimeprazine) 1mg/kg to 2mg/kg bid • Cetirizine 0.2mg/kg 0.4mg/kg bid (or 100mg per horse bid) |
| Tricyclic antidepressants* | • Amitriptyline 1mg/kg bid • Doxepin 0.5mg/kg to 0.75mg/kg bid |
| Phosphodiesterase inhibitors* | • Pentoxifylline 10mg/kg to 15mg/kg bid or tid |
| *Off-label use; po = by mouth; sid = once a day; bid = twice a day; tid = three times a day | |
Other measures that can be employed include bathing with soothing or emollient shampoos containing ingredients such as oatmeal and aloe vera extracts, and insect-avoidance measures with cases having concurrent CHS.
Unlike the disease in cats and dogs where atopic dermatitis is considered a lifelong, incurable condition, it appears that horses may develop lasting tolerance, either spontaneously or after ASIT, requiring no further treatment (Stepnik et al, 2011; Loeffler et al, 2018). In the latter study, 38.3% of cases were considered by owners to have resolved and no longer need medication. In contrast, the skin condition was unchanged or worse in spite of medication in 8.5% of cases, with euthanasia due to intractable disease resulting in 6.9% of cases. However, for the vast majority of cases, management of EAD was improved after referral.