30 Sept 2019
Equine hindlimb proximal suspensory ligament injuries
Sue Dyson reviews developments in the understanding of risk factors, and discusses diagnosis and management of this condition.

Proximal suspensory desmopathy (PSD) in hindlimbs is a challenging condition to both diagnose and manage.
This article aims to review developments in our knowledge, based on both personal experience and current literature.
Conformation as a risk factor
In a study of 193 horses with hindlimb PSD and control horses, those with PSD had larger tarsal angles than controls (p=0.003)1. The proportions of warmblood-type horses and dressage horses with PSD were larger than those of other breeds and work disciplines (p=0.001 and p=0.02, respectively).
A final logistic regression model demonstrated a significant effect of mean tarsal angle on outcome when breed and weight-height product were accounted for.
An 11% increase occurred in the odds of PSD for every degree increase in tarsal angle (confidence intervals 1.006 to 1.223; p=0.04; Figures 1 and 2a). No association existed between PSD or suspensory branch injury and metatarsophalangeal joint angle.
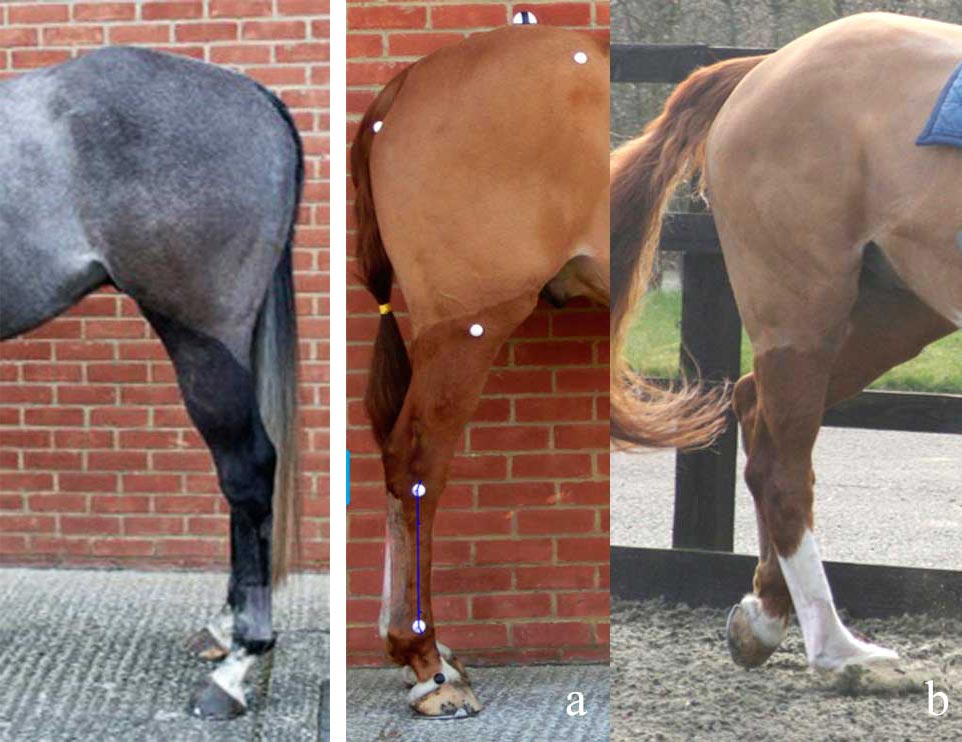
A longitudinal study would be required to determine what is cause and effect; however, purchase of a horse with tarsal angles greater than or equal to 165° is not recommended.
Surgical management of PSD – by neurectomy of the deep branch of the lateral plantar nerve, and plantar fasciotomy, in a horse with tarsal angles greater than or equal to 165° – may be associated with progressive degeneration of the suspensory ligament (SL) and persistent lameness or deterioration in lameness2.
The high prevalence of PSD in dressage horses may also reflect greater rider recognition of a compromised hindlimb gait, compared with horses from other disciplines in which quality of the gaits is potentially of less importance.
The occurrence of PSD in young warmbloods disproportionate to work history is suggestive of inherent susceptibility to injury (unpublished data).
Extension versus collection
In a study of 20 clinically sound horses in active dressage training, 10 young (six years of age and younger) were assessed at collected and medium trot, and 10 mature (nine years of age and older) were assessed at collected and extended trot7.
All horses were assessed on two different surfaces. High-speed motion capture was used to determine kinematic variables.
Speed and stride length were reduced – and stride duration increased – at collected trot, compared with medium and extended trot. Hock angle was not significantly influenced by pace. Medium or extended trot increased metatarsophalangeal joint extension, compared with collected trot, in both young and mature dressage horses, respectively.
The frequent use – or excessively long periods – of medium or extended trot may increase the risk of repetitive strain injuries of the suspensory apparatus.
Work surfaces
Clusters of horses with PSD based at specific yards have been previous observed, and it has been speculated that arena surface may be a risk for injury.
All 20 horses7 were assessed on two surfaces:
- surface A – waxed sand and fibre, which had been rolled
- surface B – unwaxed sand and rubber, which had been harrowed
No effect of arena surface type was observed in the final multivariable models. This may be due to the effect of small sample size, horses accustomed to being worked on these types of surfaces, or that kinematic values were only determined at midstance. At midstance, the limb is being fully loaded and the base of the surface influences a lot of the characteristics the horse experiences8.
Characteristics such as cushioning, grip and dampening will have more influence at impact and push-off1,8, and these may influence the kinematics of the horse at this point of the stride. The surfaces evaluated did have differences in surface characteristics (unpublished data), but these differences were more likely to affect the top of the surface and the horse’s interaction with it at impact and push-off.
Epidemiological and clinical data has suggested injury risk is linked to arena surface characteristics, such as unevenness and poor maintenance9,10.
Further investigation is required to evaluate the effect of surface on kinematics at different points of the stride.
Lameness characteristics
Although some horses with hindlimb PSD show a unilateral hindlimb lameness, in the majority, lameness is bilateral.
Lameness may only manifest when ridden; if symmetrical, lameness results in reduced hindlimb impulsion and engagement, and difficulties with specific movements.
Horses may variably adapt to pain by tension, reluctance to go forwards, or bolting.
Diagnostic anaesthesia
Diagnostic anaesthesia is an essential prerequisite for diagnosis, and horses should ideally be evaluated ridden both before and afterwards to assess the effect on ridden horse performance and behaviour.
It is essential to eliminate the distal aspect of the limb as a potential source of pain by performing plantar (proximal to the digital flexor tendon sheath) and plantar metatarsal (distal to the distal aspect of the second and fourth metatarsal bones) nerve blocks (a “low-four-point-block”).
Paradoxically, lameness may deteriorate. It is not necessary to block the dorsal metatarsal nerves11.
Perineural analgesia of the deep branch of the lateral plantar nerve (3ml mepivacaine, evaluated after 10 minutes) should result in substantial improvement in lameness. However, this is a non-specific block and has the potential to remove pain from the tarsal region12,13. In a small proportion of horses, perineural analgesia of the tibial nerve is required to abolish lameness.
In horses that present with symmetrical lack of hindlimb impulsion, without a lamer limb, best improvement will be seen by performing perineural analgesia of the deep branch of the lateral plantar nerve in both hindlimbs concurrently. Horses should be re-evaluated ridden in both trot and canter, and performing movements they previously found difficult.
Concurrent sacroiliac joint region pain is common, and paradoxically, the quality of canter may deteriorate despite improvement in lameness and quality of trot.
Given the non-specificity of perineural analgesia of the deep branch of the lateral plantar nerve, intra-articular analgesia of the tarsometatarsal joint (3ml mepivacaine, maximum) should be performed on a separate occasion.
Imaging and diagnosis of hindlimb PSD
Perineural analgesia of the deep branch of the lateral plantar nerve is not a specific block, so the tarsus and proximal metatarsal region should be evaluated using diagnostic imaging.
Radiography should be used to rule out other lesions that may be contributing to pain and lameness, and to identify osseous pathology associated with PSD, bearing in mind many non-lame horses have mild increased opacity of the proximolateral aspect of the third metatarsal bone.
High-quality ultrasonographic images are required for diagnosis of PSD and the SL should be evaluated in its entirety, because branch injuries may coexist even in the absence of improvement in lameness following a low-four-point-block (Figure 3).
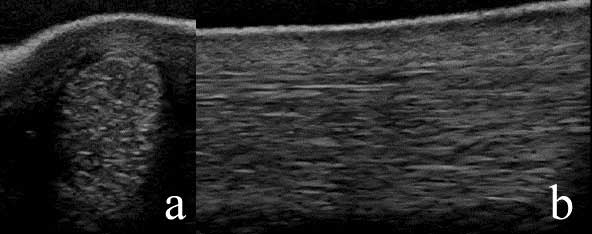
It has previously been suggested ultrasonography is unreliable for the detection of hindlimb PSD based on comparison between ultrasonographic images and MRI14. However, a more recent retrospective study was performed to compare ultrasonography with gross and histopathological postmortem examination in horses with PSD, diagnosed based on ultrasonography, and control horses15.
A good correlation existed between the presence of ultrasonographic and histological abnormalities of the SL. In part one, 19 horses with hindlimb PSD and 10 control horses were humanely destroyed. All horses with PSD had multiple problems contributing to pain and poor performance, and were humanely destroyed for reasons unrelated to the study.
Twenty control limbs and 37 lame limbs were examined grossly, and 40 SLs were examined histologically at predetermined anatomical sites and graded blindly.
Most of the horses exhibited mild to moderate lameness, which, in many horses, was only seen when ridden. Ultrasonographic lesions were graded moderate in 31 of 38 (81.6%) – and severe in 7 of 38 (18.4%) – lame limbs; in 4 of 37 limbs (10.8%), adhesion formation between the proximal aspect of the SL and the accessory ligament of the deep digital flexor tendon (ALDDFT) was predicted.
Gross postmortem and histological examinations of control limbs revealed no abnormalities. Gross postmortem examination revealed substantial adhesions between the proximal aspect of the SL and adjacent soft tissues in 10 of 37 lame limbs (27%); in 10 of 37 limbs (27%), adhesions existed between the body of the SL and the mid-plantar aspect of the third metatarsal bone, extending distally in 6 limbs (16.2%).
Histology revealed abnormalities (grades one to three) of the collagenous tissue in 25 of 36 limbs (69.4%); muscle was abnormal (grades one to three) in 35 of 36 limbs (97.2%) and adipose tissue (grades one to three) in 16 of 36 limbs (44.4%).
In one lame limb, no histological abnormalities were identified, but extensive adhesions existed between the SL and adjacent structures at gross postmortem examination.
It was concluded ultrasonography was reliable for the detection of SL pathology based on histology as the gold standard.
In part two of the study, three horses with recurrent lameness after surgical management of PSD – and four with PSD – were assessed ultrasonographically and postmortem. Adhesions between the SL and adjacent soft tissues were predicted ultrasonographically, and confirmed postmortem. The presence of such adhesions may explain why some horses fail to respond adequately to surgical treatment.
The use of off-incidence ultrasonographic imaging in a non-loaded limb may give additional information18,19, but is not essential for all horses.
Low-field MRI is not reliable for identification of suspensory pathology, but can be helpful to identify osseous causes of pain and lameness. High-field MRI is more reliable for detection of both collagen and muscle tissue pathology of the SL20, but was not superior to ultrasonography for identification of adhesions.
Knowledge of normal anatomy is an essential prerequisite for accurate interpretation21.
Skeletal scintigraphy
The presence of increased radiopharmaceutical uptake (IRU) in the proximoplantar aspect of the third metatarsal bone reflects entheseous reaction and may be a poor prognostic indicator in some horses. Its presence should alert clinicians to a careful appraisal of the horse’s hindlimb conformation.
IRU was present in the proximoplantar aspect of the third metatarsal bone in 6 of 10 limbs (60%) of five horses with tarsal angles (greater than or equal to 165°), compared with 9.6% of 270 limbs of horses with smaller hock angles – suggestive of mechanical stress at the bone-ligament interface22.
However, no significant difference existed in outcome for horses with primary PSD with or without IRU, treated by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy2, but this may reflect the small number of horses with IRU – 69% of horses with IRU had a successful outcome, compared with 78% of all horses (excluding those that developed unrelated problems).
Treatment options and prognosis
The reports of success of conservative management are variable23,24, but, in the clinical population, is usually unsuccessful.
Radial pressure wave therapy or extracorporeal shockwave therapy can be successfully used for short-term pain management associated with mild lesions25,26. Injection of biological preparations – such as platelet-rich plasma and mesenchymal stem cells – has yielded poor results. A lack of evidence-based knowledge exists about the use of class four lasers.
Neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy continues to be a highly successful method of management of hindlimb PSD, with careful patient selection – for example, resolution of lameness after perineural analgesia of the deep branch of the lateral plantar nerve (or identification of another treatable source of pain), definitive evidence of PSD and no predisposing conformational abnormalities2,27,28. A total of 78% of horses with primary PSD alone returned to full athletic function for at least one year2,28.
Pre-existing straight hindlimb conformation (hock angle greater than 165°) is a risk factor for progressive degenerative change of the ligament and failure of surgery. Horses with static or dynamic hyperextension of the metatarsophalangeal joint may also have compromised function of the suspensory apparatus and be at risk of progressive lesions (Figures 2 and 3).
Appropriate postoperative rehabilitation is helpful for best results. This should, ideally, include intensive physiotherapy and exercises designed to enhance core muscle strength, and the strength and fitness of the muscles that stabilise the hindlimbs. A small proportion of horses develop low-grade lameness after resumption of full work associated with tarsometatarsal joint pain.
The author postulates neurogenic muscle atrophy in the SL alters function; the SL and hock are biomechanically linked via the accessory ligament of the SL. Low-grade instability of the distal hock joints may predispose to pain. These horses can, generally, be successfully managed by intra-articular medication.
However, despite careful selection, a 22% failure rate still exists. Generally, these horses have persistent lameness or early recurrence of lameness, and lameness is resolved by infiltration of local anaesthetic solution around the origin of the SL or by perineural anaesthesia of the tibial nerve. Some of these failures may be due to adhesion formation, as aforementioned.
In a small number of horses, other reasons for failure have been identified. In total, 3 of 283 horses have developed proximal injuries of the ALDDFT, while 4 of 278 horses with good hindlimb conformation have developed progressive degenerative changes of the SL body within three to nine months postoperatively. One horse developed a suspensory branch injury.
Conclusion
PSD is a common cause of hindlimb lameness that, with careful patient selection, can be successfully managed.
However, neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy will not be successful for treatment of other causes of proximal plantar metatarsal region pain.
References
- Routh J, Strang C, Gilligan S and Dyson S (2019). An investigation of the association between hindlimb conformation and suspensory desmopathy in sports horses, Equine Vet Educ [Epub ahead of print], DOI: 10.1111/eve.13089.
- Dyson S and Murray RC (2012). Management of hindlimb proximal suspensory desmopathy by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy: 155 horses (2003-2008), Equine Vet J 44(3): 361-367.
- Murray RC, Dyson SJ, Tranquille C and Adams V (2006). Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis, Equine Vet J 38(Suppl 36): 411-416.
- Holmström M, Fredricson I and Drevemo S (1995). Biokinematic effects of collection on the trotting gaits in the elite dressage horse, Equine Vet J 27(4): 281-287.
- Clayton HM (1994). Comparison of the stride kinematics of the collected, working, medium and extended trot in horses, Equine Vet J 26(3): 230-234.
- Weishaupt MA, Byström A, von Peinen K et al (2009). Kinetics and kinematics of the passage, Equine Vet J 41(3): 263-267.
- Walker VA, Tranquille CA, Newton JR et al (2017). Comparison of limb kinematics between collected and lengthened (medium/extended) trot in two groups of dressage horses on two different surfaces, Equine Vet J 49(5): 673-680.
- Hobbs SJ, Northrop AJ, Mahaffey C et al (2014). Equine surfaces white paper, http://bit.ly/2kmo8RN
- Murray RC, Walters JM, Snart H et al (2010). Identification of risk factors for lameness in dressage horses, Vet J 184(1): 27-36.
- Murray RC, Walters J, Snart H et al (2010). How do features of dressage arenas influence training surface properties which are potentially associated with lameness?, Vet J 186(2): 172-179.
- Coleridge M, Schumacher J and DeGraves F (2018). Comparison of lameness scores after a low 4-point nerve block to lameness scores after additional desensitisation of the dorsal metatarsal nerves in horses with experimentally induced pain in the metatarsophalangeal joint, Equine Vet Educ [Epub ahead of print], DOI: 10.1111/eve.12942
- Dyson S and Romero J (1993). An investigation of injection techniques for local analgesia of the equine distal tarsus and proximal metatarsus, Equine Vet J 25(1): 30-35.
- Contino EK, King MR, Valdés-Martínez A and McIlwraith CW (2015). In vivo diffusion characteristics following perineural injection of the deep branch of the lateral plantar nerve with mepivacaine or iohexol in horses, Equine Vet J 47(2): 230-234.
- Labens R, Schramme MC, Robertson ID et al (2010). Clinical, magnetic resonance and sonographic findings in horses with proximal plantar metatarsal pain, Vet Radiol Ultrasound 51(1): 11-18.
- Dyson S, Murray R and Pinilla M (2017). Proximal suspensory desmopathy in hindlimbs: a correlative clinical, ultrasonographic, gross post mortem and histological study, Equine Vet J 49(1): 65-72.
- Dyson S (2014). Hindlimb lameness associated with proximal suspensory desmopathy and injury of the accessory ligament of the suspensory ligament in five horses, Equine Vet Educ 26(10): 538-542.
- Plowright E and Dyson S (2015). Concurrent proximal suspensory desmopathy and injury of the proximal aspect of the accessory ligament of the deep digital flexor tendon in forelimbs or hindlimbs of 19 horses, Equine Vet Educ 27(7): 355-364.
- Werpy NM, Denoix JM, McIlwraith CW and Frisbie DD (2013). Comparison between standard ultrasonography, angle contrast ultrasonography, and magnetic resonance imaging characteristics of the normal proximal suspensory ligament, Vet Radiol Ultrasound 54(5): 536-547.
- Denoix JM and Bertoni L (2015). The angle contrast ultrasound technique in the flexed limb improves assessment of proximal suspensory ligament injuries in the equine pelvic limb, Equine Vet Educ 27(4): 209-217.
- Dyson S, Pinilla MJ, Bolas N and Murray R (2018). Proximal suspensory desmopathy in hindlimbs: magnetic resonance imaging, gross post mortem and histological study, Equine Vet J 50(2): 159-165.
- Dyson S, Blunden A and Murray R (2017). Magnetic resonance imaging, gross postmortem, and histological findings of the soft tissues of the plantar aspect of the tarsus and proximal metatarsal region in non-lame horses, Vet Radiol Ultrasound 58(2): 217-227.
- Dyson SJ, Weekes JS and Murray RC (2007). Scintigraphic evaluation of the proximal metacarpal and metatarsal regions of horses with proximal suspensory desmitis, Vet Radiol Ultrasound 48(1): 78-85.
- Dyson S (1994). Proximal suspensory desmitis in the hindlimb: 42 cases, Br Vet J 150(3): 279-291.
- Norvall A, Allen K, Johns S et al (2015). Diagnosis, treatment and outcome of hindlimb proximal suspensory desmopathy in sport horses: 75 cases (2008-2014), Proceedings of the 61st Annual Convention of the American Association of Equine Practitioners, Las Vegas: 358.
- Crowe OM, Dyson SJ, Wright IM et al (2004). Treatment of chronic or recurrent proximal suspensory desmitis using radial pressure wave therapy in the horse, Equine Vet J 36(4): 313-316.
- Lischer CJ, Ringer SK, Schnewlin M et al (2007). Treatment of chronic proximal suspensory desmitis in horses using focused electrohydraulic shock wave therapy, Schweizer Arch Tierheilkd 148(10): 561-568.
- Kelly G (2007). Results of neurectomy of the deep branch of the lateral plantar nerve for treatment of proximal suspensory desmitis, Proceedings of the 16th Annual Convention of the European College of Veterinary Surgeons, Dublin: 130.
- Bathe A (2017). Surgical management of proximal suspensory desmitis, Proceedings of the 56th Annual Congress of the British Equine Veterinary Association, Birmingham: 71.
- Barstow A and Dyson S (2015). Clinical features and diagnosis of sacroiliac joint region pain in 296 horses: 2004-2014, Equine Vet Educ 27(12): 637-647.
