5 Aug 2025
Equine metabolic syndrome

Image: Duncan Noakes / Adobe Stock
Equine metabolic syndrome (EMS) is a collection of metabolic and clinical features resulting in an increased risk of hyperinsulinaemia-associated laminitis in horses and ponies.
The key central and consistent feature of EMS is insulin dysregulation (ID), which can manifest in an individual animal as any combination of basal (resting) hyperinsulinaemia, an excessive insulin response to an oral carbohydrate challenge and/or tissue insulin resistance.
Additional features of EMS are inconsistent and comprise obesity, which may be generalised or regional; cardiovascular changes including increased blood pressure, heart rate and cardiac dimensions; and adipose dysregulation manifesting as abnormal plasma adipokine concentrations, including hypoadiponectinaemia and hyperleptinaemia, and/or increased fasting serum triglyceride concentrations.
Causes of ID in animals with EMS
The exact mechanism(s) underlying ID in EMS remain uncertain; however, evidence suggests that several mechanisms might play a role, including the following.
Breed
Some breeds have an increased risk of developing ID. At-risk breeds include UK native pony breeds, Iberic breeds (Andalusians), gaited breeds (Saddlebreds, paso finos), Morgans, miniature horses, Arabian horses and warmbloods.
Genetics
Horses with EMS are often considered to be “good doers” or “easy keepers”, and genes contributing to this phenotype are likely to have been advantageous for survival in the wild during periods of famine by enhancing feed efficiency.
Initial research found that the prevalence of EMS-associated laminitis was consistent with the action of a major gene or genes expressed dominantly, but with reduced penetrance attributable to sex-mediated factors, age of onset and further epigenetic factors1.
More recently, genome-wide association studies (GWAS) involving Arabian horses, Welsh ponies and Morgan horses have identified several candidate genes that were associated with traits relevant to EMS, including height and insulin, triglyceride and adiponectin concentrations. It appears that the genetic risk factors for EMS may differ between equine breeds, and it is likely that the disease is genetically multifactorial, with multiple alleles underlying genetic susceptibility.
In addition, the contribution of currently identified genes seems limited compared to that of the far greater contribution of environmental factors.
Epigenetics
The environment in utero influences the metabolic health of the fetus later in life. Certain genes are switched on or off depending on the nutritional status of the mother, which then influence the offspring’s metabolic health over time.
Both maternal overnutrition and undernutrition appear to increase the risk of obesity and of being metabolically unhealthy in people, and limited evidence exists to support the supposition that this also occurs in horses2.
Age
While in one study basal insulin concentrations were not different between younger and older animals3, in other studies concentrations were significantly higher in older healthy horses4 and ponies5. These age-related differences have also been demonstrated in studies evaluating insulin responses to an intravenous glucose challenge6, an oral glucose challenge7 and to feeding8.
Obesity
It is now recognised that obesity is not a central feature of EMS; instead, it is an additional feature seen in some, but not all animals.
The prevalence of obesity has been reported in numerous subpopulations worldwide, ranging from 21% to 45% in various UK horse and pony subpopulations9-13. Adipose tissue is the largest endocrine organ in the body, producing an array of hormones with normal physiological roles.
Obesity results in adipose dysregulation, such that the production of some hormones is increased (such as leptin) and others is decreased (such as adiponectin). Some of the affected hormones impact insulin sensitivity, with the overall result being insulin dysregulation.
Diet
Diets high in non-structural carbohydrate will reduce insulin sensitivity and adiponectin concentrations in horses compared to forage or fat-rich diets14,15.
Gut microbiome
The gastrointestinal microbiome influences metabolism, endocrine signalling and the immune system in humans; for example, perturbations of the faecal microbiome in humans and mice have been associated with alterations in insulin secretion and sensitivity, incretin action and obesity (that is, a microbe-gut-endocrine axis).
In horses, changes in gut flora have been implicated in the pathogenesis of laminitis16, and horses with EMS have decreased gastrointestinal microbial diversity and differences in community structure compared to non-EMS animals17,18.
Conclusion: risk factors for EMS
Since ID is the central feature of EMS, the risk factors for EMS are identical to the possible causes of ID, only some of which are modifiable.
While breed, age, individual animal genetics and epigenetics cannot be influenced, obesity, diet and the gastrointestinal microbiome can be altered to impact insulin sensitivity.
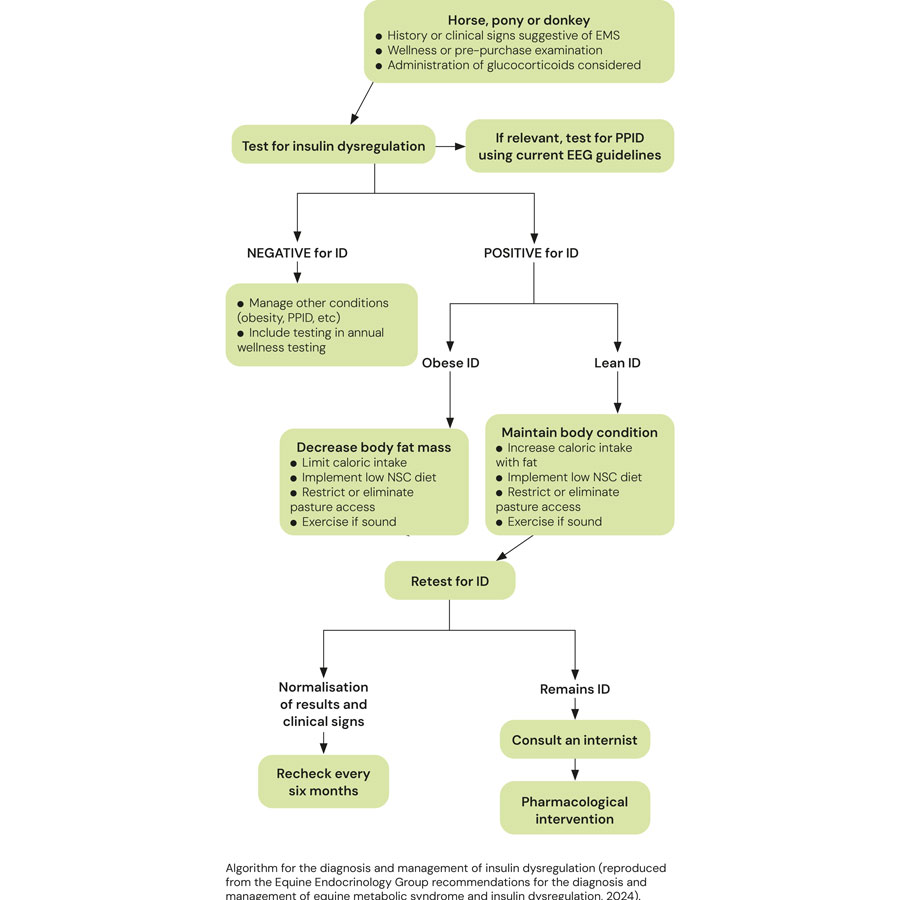
Diagnosis of EMS
Diagnosis of EMS requires the detection of ID.
Identification of some of the additional features of EMS provides additional supportive evidence.
Detection of ID
ID in the horse manifests in three forms, namely hyperinsulinaemia, an excessive insulin response to oral carbohydrate and peripheral (tissue) insulin resistance.
Any combination of these manifestations may occur in an individual animal; therefore, it is important to consider testing for all three.
- Basal insulin concentrations
Hyperinsulinaemia is detected by measuring basal insulin concentrations in a single blood sample collected with the horse in the fed state (hay or pasture, but not grain).
Higher resting insulin concentration indicates higher laminitis risk; although, if the resting insulin concentration is normal, a dynamic test still must be performed before ID can be ruled out.
- Oral sugar test
An oral sugar test (OST) is a dynamic test recommended to detect an excessive insulin response to carbohydrate. The OST is the preferred dynamic test because the insulin concentrations measured reflect a more complete sequence of events, including digestion and absorption of sugars, incretin hormone responses and secretion of insulin from the pancreas.
Advantages of this test include the ready availability of commercial corn syrup, ease of administering corn syrup, and the test’s assessment of insulin responses to ingested sugars.
Disadvantages include the requirement for horses to ideally be fasted for three to six hours prior to testing; however, fasting conditions are often achieved by the owner leaving one flake/slice of hay with the horse before midnight and then the test is performed the following morning. Alternatively, the test can be performed while the horse remains at pasture. In situations where horses are resistant to oral administration, the corn syrup can be mixed with a small amount of low-glycaemic feed (such as chaff).
Oral glucose dextrose powder can alternatively be used (oral glucose test) if a commercial corn syrup is not available and this should also be mixed with a small amount of low-glycaemic feed. See Table 1 for details relating to the procedure and interpretation of the results.
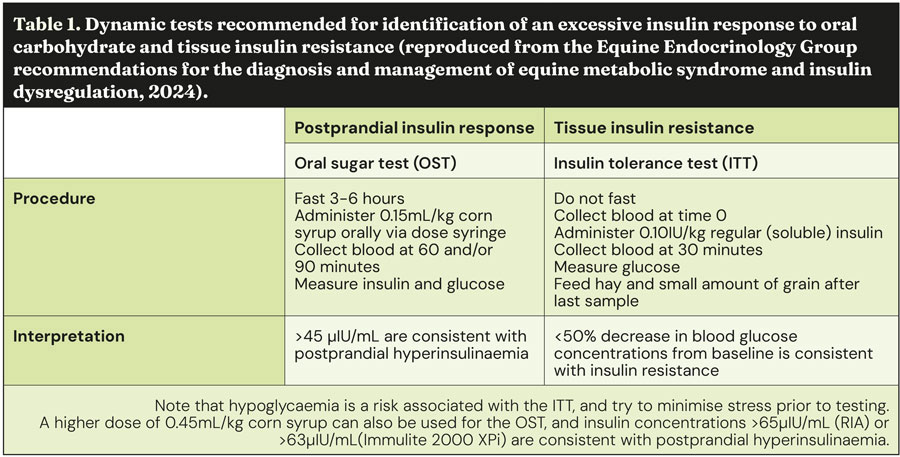
- Insulin tolerance test
The insulin tolerance test (ITT) is recommended to detect peripheral (tissue) insulin resistance. Advantages of the ITT are that this test does not require pre-test fasting and blood glucose concentrations can be measured with a handheld glucometer, so preliminary results are available on the farm.
Disadvantages include the cost of purchasing the insulin and the very small risk of clinical hypoglycaemia developing during the test. Horses should be monitored for the duration of the test, and hay and a small amount of grain should be fed immediately after the 30-minute sample is collected to further mitigate hypoglycaemia risk. See Table 1 for further details relating to the procedure and interpretation of the results.
Identification of additional features of EMS
Concentrations of the adipokine adiponectin are decreased in a proportion of animals with EMS and associated with an increased risk of future laminitis independent of insulin concentration; therefore, measurement provides additional information relating to laminitis risk.
Circulating triglyceride concentrations are increased in some animals with EMS, and so should be measured in animals in which sodium glucose-like transporter-2 inhibitors (SGLT2i) therapy is being considered due to hypertriglyceridaemia being a potential side effect.
Rule out pituitary pars intermedia dysfunction
ID occurs in a subset of animals with pituitary pars intermedia dysfunction (PPID) and this same subset has an increased risk of laminitis; therefore, it is prudent to rule in/out PPID in animals with ID that are older than seven years of age by measuring basal adrenocorticotropic hormone concentrations and/or performing a thyrotropin-releasing hormone stimulation test.
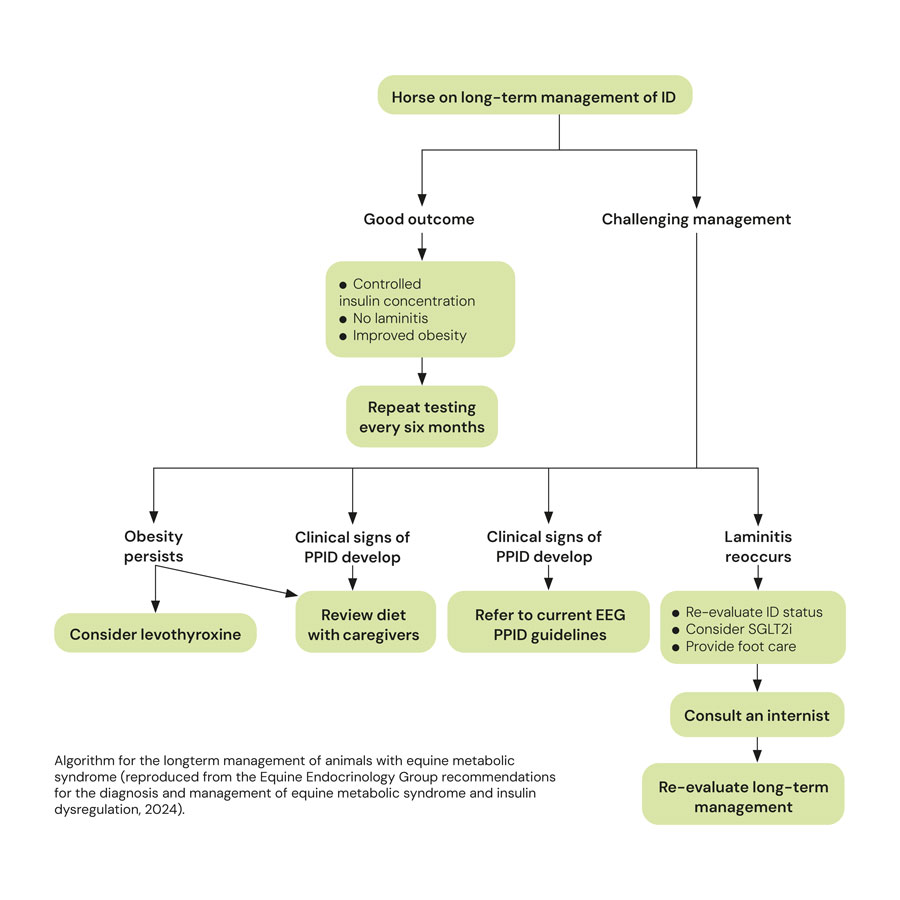
Management of EMS
The management of EMS consists of dietary modification, exercise and the short-term use (three to six months) of pharmacologic agents, in some cases.
Diet
Dietary modification recommendations depend on whether the animal is obese or lean.
Obese animals
Obesity is managed primarily via energy restriction through limiting intake. An ideal target for weight loss is 0.5% to 1.0% of body mass (BM) weekly. A daily allowance of 1.25% to 1.5% of actual BM as dry matter intake (DMI), or 1.4% to 1.7% of actual BM as fed, is widely recommended. In horses with weight loss resistance, a further forage restriction to 1.0% BM as DMI or 1.15% BM as fed may be considered if appropriately monitored.
Grains or cereal-based feeds should be excluded due to their high content of non-structural carbohydrate (NSC), and high-fat feeds should be avoided due their high energy content. Additionally, large amounts of fruit or vegetables such as carrots, apples or treats with a high glycaemic load should be avoided.
The nutrient composition of the forage should ideally be determined where possible, and hays with low NSC content (less than 10%) are recommended to limit postprandial insulin responses. Soaking for one hour in warm water or six hours in cold water is advised to reduce the NSC content of the hay if necessary. Straw is a cost-effective, low-energy forage that may be used as an alternative to some of the hay ration. As forages can be low in protein, and mineral and vitamin leaching occurs after soaking, these nutrients must be balanced by low-calorie supplements to cover requirements.
During the initial 6 to 12 weeks of dietary restriction, pasture access should be prevented, as even partial access is very difficult to quantify. However, successful long-term management of EMS cases can still include some grazing provided that the ID – especially assessed by the insulin response to oral carbohydrates or grazing – is under control and grazing is also carefully controlled. The use of dietary supplements such as cinnamon, magnesium and chromium to facilitate weight loss or to improve ID is popular, but their efficacy remains questionable or unproven.
Lean animals
Lean animals should be fed a low glycaemic diet to minimise the postprandial insulin response.
The diet should be based on forage with a low (ideally less than 10%) NSC content and additional calories provided in the form of fat (such as vegetable oil) and high-quality fibre such as beet pulp. A low-calorie vitamin, mineral and protein ration balancer should be fed as required.
Exercise
Exercise has been shown to improve insulin sensitivity in people; however, studies in horses have shown variable results. Exercise should only be considered in animals without current laminitis and should be gradually increased based on fitness.
The optimal exercise regime has yet to be determined; however, half-hour sessions of exercise which raise the heart rate to more than 150bpm, performed five days per week, are recommended.
Pharmacologic management
The class of pharmacologic agent that has received most attention recently is the SGLT2i.
They are advocated to aid a reduction in circulating insulin concentrations in cases where diet and exercise alone are not successful, or in cases with acute laminitis to improve lameness.
Use of SGLT2 inhibitors in humans
Obesity, ID, hypoadiponectinaemia, hyperleptinaemia and an altered plasma lipid profile are features of human metabolic syndrome (HMS)19, as well as EMS. SGLT2i is a novel class of oral hypoglycaemic agent used in combination with lifestyle changes in the management of HMS.
SGLT2 receptors are responsible for 90 per cent of the renal glucose reabsorption that occurs in the proximal convoluted tubule. Subsequently, these drugs increase urinary glucose excretion by suppressing glucose reabsorption from the glomerular filtrate, resulting in urinary glucose and, therefore, calorie loss with consequent weight loss and improvements in ID, hyperglycaemia, hypoadiponectinaemia and hyperleptinaemia20,21. The overall result is significant cardiorenal protective effects22. While some of these beneficial effects can be explained by improved glycaemic control, other mechanisms are thought to be involved; natriuresis (as sodium is lost alongside the glucose) with a reduction in plasma volume, a consequent rise in haematocrit, improved vascular function, a reduction in blood pressure and changes in tissue sodium handling are all likely to have a role23.
Additional mechanisms of SGLT2i that might be beneficial in people include a reduction in adipose tissue-mediated inflammation and pro-inflammatory cytokine production, a shift towards ketone bodies as the metabolic substrate for the heart and kidneys, reduced oxidative stress, lowered serum uric acid level, reduced glomerular hyperfiltration and albuminuria, and suppression of advanced glycation end-product signaling23.
SGLT2i in horses
No licensed veterinary drugs are available for treating ID and preventing insulin-associated laminitis in horses; therefore, the use of the SGLT2i used in HMS has recently been advocated for the control of equine hyperinsulinaemia, with the goal of improving recovery from associated active laminitis or preventing future laminitis24-26.
The drugs reportedly used to date include velagliflozin27,28, canagliflozin24,26,29,30, ertugliflozin25,31, dapagliflozin32 and empagliflozin (anecdotal reports). All of these are used in humans apart from velagliflozin, which is licensed for the treatment of diabetes mellitus in cats not previously treated with insulin33.
Canagliflozin (0.3mg/kg to 0.6mg/kg once daily orally)24,26,30,34 has been shown to lower serum insulin concentrations, correct hyperglycaemia, reduce abnormal fat pads and eliminate laminitis pain. The time to resolution of laminitis lameness ranged from 24 hours to several weeks. However, increases in insulin were observed when the concomitant PPID was not controlled, or the diet was liberalised24, and insulin concentrations did not return to normal in all animals30. In addition, significant increases were noted in triglyceride concentrations in some animals.
Use of ertugliflozin (0.05mg/kg once daily orally) in the management of hyperinsulinaemia and laminitis in 51 horses was associated with a reduction in circulating insulin concentrations and lameness grade associated with laminitis after 30 days25. Insulin concentrations remained low through subsequent follow-up tests in most horses, but in 8 out of 51, an increase in insulin was identified beyond 30 days of treatment, potentially due to owners failing to adhere to the concurrent long-term diet and management recommendations. In a second study, 10 horses received four once-daily oral doses of ertugliflozin (0.05mg/kg) and this was associated with lowering of insulin concentrations at baseline and in response to an OST; however, insulin concentrations did not return to normal in all horses31.
Velagliflozin is only commercially available in an oral formulation for cats; however, it has been used in equine experimental studies. Following a four-week, placebo-controlled trial treatment (n=4 control and n=4 treated horse; 0.1mg/kg to 1mg/kg once daily orally), glucose and insulin responses to an OST, plasma leptin concentrations and bodyweight were all reduced35. In a second study, treatment with velagliflozin (0.3mg/kg once daily orally for three weeks) lowered postprandial insulin and glucose concentrations and prevented laminitis development in 12 ponies with ID, but normoglycaemia, which were fed a challenge diet high in NSC for up to 18 days, compared to 37 similar but untreated ponies27. In a third study, 24 ponies with ID and normoglycaemia were treated with velagliflozin at the same dose for 16 weeks and fed a maintenance diet28. Postprandial serum insulin concentrations were significantly decreased at week 16, but not week eight, and had returned to pre-treatment values four weeks after withdrawal of treatment with no rebound effect; bodyweight remained unchanged. While anecdotal reports exist of using empagliflozin to aid management of ID in horses, no reports have been published in the scientific literature. A single publication has reported use of dapagliflozin (0.02mg/kg once daily orally) in 34 horses32; basal insulin concentrations, modified Obel lameness grade, bodyweight, body condition score and cresty neck score all significantly decreased from day 0 to day 7 and day 30.
Side effects of SGLT2i therapy in horses
Use of SGLT2i in horses is associated with hypertriglyceridaemia24,25,30,31. This appears to be mild in most cases, not associated with triglyceride-induced renal or hepatic dysfunction, and not linked to any treatment-associated weight loss, but in isolated cases may be severe34.
Additionally, it appears to occur predominantly in the short term and mostly improves with continued therapy25. The other side effect reported to be associated with SGLT2i use in horses is polyuria and polydipsia25.
- Use of some of the drugs in this article is under the veterinary medicine cascade.
- Article appeared in Vet Times (2025), Volume 55, Issue 30, Pages 12-15
References
- 1. Treiber KH et al (2006). Evaluation of genetic and metabolic predispositions and nutritional risk factors for pasture-associated laminitis in ponies, J Am Vet Med Assoc 228(10): 1,538-1,545.
- 2. Robles M et al (2018). Maternal obesity increases insulin resistance, low-grade inflammation and osteochondrosis lesions in foals and yearlings until 18 months of age, Plos One 13(1): e0190309.
- 3. Kawasumi K et al (2015). Aging effect on plasma metabolites and hormones concentrations in riding horses, Open Vet J 5(2): 154-157.
- 4. Hart KA et al (2016). Effect of age, season, body condition, and endocrine status on serum free cortisol fraction and insulin concentration in horses, J Vet Intern Med 30(2): 653-663.
- 5. Morgan RA et al (2014). Prevalence and risk factors for hyperinsulinaemia in ponies in Queensland, Australia, Aust Vet J 92(4): 101-106.
- 6. Liburt NR et al (2011). The effect of exercise training on insulin sensitivity and fat and muscle tissue cytokine profiles of old and young Standardbred mares, J Equine Vet Sci 31(5-6): 237-238.
- 7. Malinowski K et al (2002). Effect of training on age-related changes in plasma insulin and glucose, Equine Vet J Suppl 34: 147-153.
- 8. Nielsen BD et al (2010). Glycemic and insulinemic responses are affected by age of horse and method of feed processing, J Equine Vet Sci 30(5): 249-258.
- 9. Stephenson HM et al (2011). Prevalence of obesity in a population of horses in the UK, Vet Rec 168(5): 131.
- 10. Harker IJ et al (2011). The body condition score of leisure horses competing at an unaffiliated championship in the UK, J Equine Vet Sci 31(5): 253-254.
- 11. Giles SL et al (2014). Obesity prevalence and associated risk factors in outdoor living domestic horses and ponies, PeerJ 2: e299.
- 12. Robin CA et al (2015). Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores, Equine Vet J 47(2): 196-201.
- 13. Wyse CA et al (2008). Prevalence of obesity in riding horses in Scotland, Vet Rec 162(18): 590-591.
- 14. Pratt SE et al (2006). Effects of dietary energy source and physical conditioning on insulin sensitivity and glucose tolerance in standardbred horses, Equine Vet J Suppl 36: 579-584.
- 15. Bamford NJ et al (2016). Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals, Vet J 214: 14-20.
- 16. Milinovich GJ et al (2010). Microbial events in the hindgut during carbohydrate-induced equine laminitis, Vet Clin North Am Equine Pract 26(1): 79-94.
- 17. Steelman SM et al (2012). Pyrosequencing of 16S rRNA genes in fecal samples reveals high diversity of hindgut microflora in horses and potential links to chronic laminitis, BMC Vet Res 8: 231.
- 18. Elzinga SE et al (2016). Comparison of the fecal microbiota in horses with equine metabolic syndrome and metabolically normal controls fed a similar all-forage diet, J Equine Vet Sci 44: 9-16.
- 19. Reaven G (2002). Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease, Circulation 106(3): 286-288.
- 20. Wang Y and Xia N (2022). Influence of sodium-glucose cotransporter-2 inhibitors on plasma adiponectin in patients with type 2 diabetes: a meta-analysis of randomized controlled trials, Horm Metab Res 54(12) :833-844.
- 21. Wu P et al (2019). Systematic review and meta-analysis of randomized controlled trials on the effect of SGLT2 inhibitor on blood leptin and adiponectin level in patients with type 2 diabetes, Horm Metab Res 51(8): 487-494.
- 22. Akiyama H et al (2023). Evolution of sodium-glucose co-transporter 2 inhibitors from a glucose-lowering drug to a pivotal therapeutic agent for cardio-renal-metabolic syndrome, Front Endocrinol 14: 1111984.
- 23. Cowie MR and Fisher M (2020). SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control, Nat Rev Cardiol 17(12): 761-772.
- 24. Kellon EM and Gustafson KM (2022). Use of the SGLT2 inhibitor canagliflozin for control of refractory equine hyperinsulinemia and laminitis, Open Vet J 12(4): 511-518.
- 25. Sundra T et al (2022). Preliminary observations on the use of ertugliflozin in the management of hyperinsulinaemia and laminitis in 51 horses: a case series, Equine Vet Educ 35(6): 311-320.
- 26. Frank N (2018). Safety and efficacy of canagliflozin and octreotide for managing insulin dysregulation in horses, J Vet Intern Med 32(6): 2,123-2,143.
- 27. Meier A et al (2018). The sodium-glucose co-transporter 2 inhibitor velagliflozin reduces hyperinsulinemia and prevents laminitis in insulin-dysregulated ponies, Plos One 13(9): e0203655.
- 28. Meier A et al (2019). The efficacy and safety of velagliflozin over 16 weeks as a treatment for insulin dysregulation in ponies, BMC Vet Res 15(1): 65.
- 29. Michanek P et al (2023). The effect of canagliflozin on blood glucose and insulin in healthy standardbred horses, J Vet Pharmacol Ther 46(1): 79.
- 30. Lindåse S et al (2023). Short-term effects of canagliflozin on glucose and insulin responses in insulin dysregulated horses: a randomized, placebo-controlled, double-blind, study, J Vet Intern Med 37(6): 2,520-2,528.
- 31. Sundra T, Rossi G, Kelty E, et al. Oral sugar test responses to ertugliflozin in ten horses with insulin dysregulation, Equine Vet Educ 36(6): 317-324.
- 32. Sundra T et al (2025). Short-term clinical and biochemical responses following treatment with dapagliflozin or ertugliflozin in horses with hyperinsulinemia: a retrospective case series, Domest Anim Endocrinol 90: 106894.
- 33. Hoenig M et al (2018). Effects of the sodium-glucose cotransporter 2 (SGLT2) inhibitor velagliflozin, a new drug with therapeutic potential to treat diabetes in cats, J Vet Pharmacol Ther 41(2): 266-273.
- 34. Kellon EM and Gustafson KM (2023). Hypertriglyceridemia in equines with refractory hyperinsulinemia treated with SGLT2 inhibitors, Open Vet J 13(3): 365-375.
- 35. Reiche D et al (2015). Treatment of metabolic disorders in equine animals, European Patent Office, tinyurl.com/49v33epu
