11 Nov 2019
Equine obesity: the most common risk factor for laminitis in the UK?
David Rendle, Pat Harris and Nicola Menzies-Gow discuss the welfare threat posed by this issue, and the need for education to alter perceptions of healthy body condition.
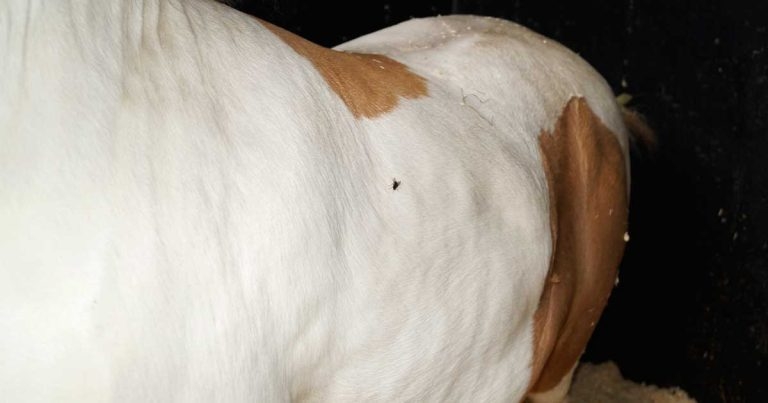
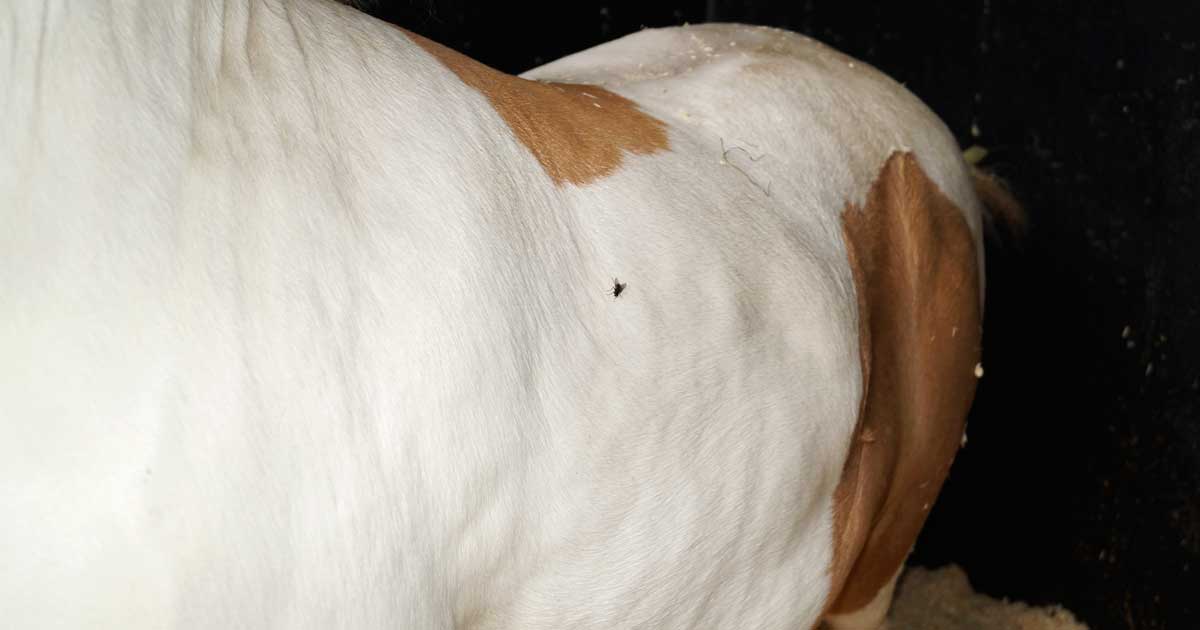
Figure 1. Large SC fat deposits in a generally obese native horse.
Obesity is often cited as the greatest threat to equine welfare in the UK. Rates may be as high as 70% in some populations1 and the condition can be associated with morbidities, most notably laminitis, that may ultimately result in mortality.
The rise of obesity in horses mirrors the situation in companion animals that was reported by Veterinary Times in July (VT49.28):
Equine metabolic syndrome (EMS) describes a conflation of metabolic disturbances that has insulin dysregulation as its foundation, and is typically associated with generalised or localised obesity (although can be found in the lean animal) and may ultimately lead to laminitis. Although other mechanical mechanisms by which laminitis may develop have been reported, EMS is by far the most common cause of pasture-associated laminitis.
Additionally to EMS and laminitis, adverse consequences of obesity include orthopaedic disease, hyperlipaemia, hyperthermia/heat intolerance, increased respiratory effort, infertility and poor performance.
Many factors are likely to have contributed to the apparent increase in prevalence of obesity, including a change in perception of what constitutes a healthy body condition, increasingly sedentary lifestyles, excess energy intake (including from grass), provision of inappropriate feedstuffs/forages, and over-rugging.
Defining obesity and EMS
Horses with a body condition score (BCS) of seven or higher – using a nine-point scale – are considered to be obese2,3 as fat is likely to account for more than 20% of bodyweight (BWT). In very obese horses, fat mass may even exceed 40% of BWT.
While a definition of obesity as a BCS of seven or higher is crude, it is widely used. Using a five-point scale, horses with a BCS higher than three are considered obese.
Leisure horses should, ideally, have a BCS of five tending to six towards the end of summer, and five tending to four at the end of winter; owners should aim for corrective weight loss over winter.
Obesity is the excessive (pathological) accumulation of fat to the point at which a negative health impact occurs on the individual. It may be generalised (Figure 1) or focal (regional), external (palpable SC deposits) or internal (covert accumulations of adipose tissue within and around organs and muscle).
The pathological processes that may develop with excess adiposity include inflammation, oxidative stress, stimulation of the hypothalamic-pituitary-adrenal axis, disturbances of cortisol – as well as lipid – metabolism, and vascular dysfunction.
EMS describes the presence of insulin dysregulation (ID) with or without other metabolic alterations and is associated with an increased risk of laminitis. Obesity is typically present, but is not a defining feature.
Strictly, diagnosis of EMS necessitates demonstrating that ID is present, either by identifying hyperinsulinaemia or abnormal insulin responses to an oral or IV sugar challenge.
A presumptive diagnosis of EMS is often made if obesity and laminitis are concurrent, and the laminitis has no other apparent cause. Some animals, particularly native types, will always be at an increased risk of laminitis if they are fed inappropriately.
Obesity and ID are often overlooked in older, leaner equids, which have a tendency towards intra-abdominal fat deposition. In these cases, laminitis may erroneously be attributed to pituitary pars intermedia dysfunction (PPID) if appropriate investigations are not performed.
Consequences of obesity
Obesity and recent weight gain have been demonstrated to have a number of negative consequences on health, including:
- increased risk of laminitis4
- poorer prognosis for recovery from laminitis5
- increased risk of hyperlipaemia6
- impairment of normal thermoregulation7
- altered oestrous cycles and decreased fertility8
- increased proinflammatory cytokine production characteristic of “inflamm-ageing”9
- greater risk of osteochondrosis dissecans in foals born to obese mares10
- undesirable behavioural traits11
- increased blood pressure12
- It may also be associated with:
- Increased risk of orthopaedic disease through increased loading.
- Preputial and mammary oedema and dermatitis.
- Ventral oedema, possibly as a consequence of compromised lymphatic drainage.
- Strangulating small intestinal lesions caused by pedunculated mesenteric lipomas.
- Greater susceptibility to hypertriglyceridaemia and hyperglycaemia when faced with other critical illness.
- Inappropriate lactation, possibly via effects on thermoregulation and prolactin production.
- Subfertility in mares and stallions.
- Reduced growth rates in foals caused by excessive mammary adiposity and reduced milk production, and/or exaggerated compensatory growth rates once weaned.
- Respiratory compromise13. Relative to total body mass, the mass of the respiratory tract may decrease as much as 15%2.
- Pharyngeal collapse.
- Poor performance14.
Laminitis and EMS often go unrecognised by owners in breeds that are susceptible to obesity. Clinical signs of acute laminitis can be attributed to other causes of foot pain or lameness, and signs of chronic disease – such as irregular horn growth – are often missed or attributed to nutritional changes15.
The presence of irregular growth rings – which typically diverge towards the heel – is extremely common in native ponies and likely indicates prior episodes of laminitis16, which may have been associated with pain even if this was not noted by the owner.
Episodes of mild or subclinical laminitis are likely to render the affected animal more susceptible to more severe laminitis in the future.
Identifying obesity
- BWT is often used as a proxy for obesity. Portable weighbridges are extremely useful, but should be calibrated regularly.
- Weigh tapes may be helpful, but have not been validated for obese horses and take no account of focal adiposity. Changes in girth will tend to lag behind changes in BWT.
- Different body condition scoring schemes have been proposed, with the nine-point scale – modified in 199217 from the original in 198318 – being the most widely accepted and studied. At the top of the body condition scoring scale, less discrimination of body fat content exists; however, horses of a BCS of seven or higher are considered obese3. BSC is less precise when used to assess UK native breeds13, which are mostly overweight, and can be very slow to change in response to reductions in BWT19. Morphometric measurements typically change before BCS in such cases7,19.
- Crest neck score20 and crest depth21,22 provide estimates of nuchal fat deposition, and insulin resistance has been associated with neck crest adiposity in some studies12,23. However, the neck crest fat (Figure 2) is functionally discrete, heavily influenced by breed, and develops and depletes more slowly than other fat deposits24,25. Furthermore, changes in nuchal crest adiposity reflect longer-term management trends, may be affected by season and may not correlate with generalised obesity25. Once a nuchal crest develops, it is unlikely to disappear as connective tissue will remain. Belly girth (Figure 3) provides the most sensitive indicator of generalised fat loss in response to management change and is arguably the single most useful measure for monitoring; heart girth (Figure 4) and rump width may also be used (Figure 5)19,26.
- Ultrasonography is a useful means of demonstrating the presence of fat, although it is a poor means of determining total body fat composition. Fat depth can be measured most easily on the ventral midline immediately caudal to the xiphisternum and about 10cm either side of the linea alba.
- Serum adiponectin concentration decreases with increasing adiposity, although this is breed and diet-dependent27. A low total adiponectin concentration is associated with increased risk of laminitis28-30, such that adiponectin is a potentially useful marker of pathological adiposity. Of the tests offered in the UK, only an immunoturbidimetric assay for total adiponectin has been shown to be reliable and correlate with laminitis risk; other methods generate inconsistent results30.
- Measures of insulin dynamics do not give an indication of obesity, per se, but are a useful means of assessing the risk of laminitis, which may be associated with obesity and metabolic dysfunction. Basal insulin concentrations provide an indication of insulin dysregulation if they are increased; however, the test has low sensitivity, so a normal result does not rule out ID. Tests of insulin responses to oral sugars provide a more sensitive means of identifying ID and are, therefore, recommended. The high-dose oral sugar test is simple to perform and can overcome issues of palatability, which can occur when glucose or dextrose powder are used as the sugar challenge. Horses should ideally be fasted for 3 to 12 hours (although this is not imperative), administered 45ml of corn sugar per 100kg BWT by mouth using a dosing syringe, then have two blood samples collected at 10 to 15-minute intervals between 60 and 90 minutes after the sugar was administered31.
- PPID is a risk factor for laminitis and may occur concurrently with obesity. Obesity and EMS can be overlooked when PPID is diagnosed, and it is important ID is identified and managed additionally, providing therapy for pituitary dysfunction. Effective management of ID will have a more direct effect on reducing laminitis risk than alterations in pituitary function. Obesity will increase the activity of the hypothalamic-pituitary-adrenal axis and increase adrenocorticotropic hormone (ACTH) concentration in horses that do not have PPID32 – so a danger exists that obesity-related laminitis is mistaken for PPID. More conservative guidelines for the diagnosis of PPID have been recommended33 and obese horses with mild increases in ACTH concentration should be appraised critically before they are treated with pergolide. Application of the thyrotropin-releasing hormone stimulation test and titration of the dose of pergolide, in response to diagnostic testing, aids in differentiating horses with PPID from those with obesity and EMS.
Feeding obese equids
A reduction in BWT of between 0.5% and 1% per week is a realistic target, but programmes of weight loss should be tailored to the patient and consider what is achievable for the owner.
Greater weight loss is likely to occur for the first week after dietary restriction as a result of reduced gut fill. Current BWT is generally used, rather than target weight, and this should be remeasured or recalculated regularly (preferably weekly) and diet adjusted accordingly.
Discontinuation of overfeeding and provision of more appropriate feed sources may be sufficient to induce weight loss in some animals.
In patients with obesity and laminitis, weight loss programmes ought to be more aggressive, as an increased risk exists of future laminitis and a need to limit hyperinsulinaemia. Furthermore, exercise cannot be included as part of the management programme in horses with laminitis.
Access to pasture is always desirable as it allows expression of normal behaviour; however, when pasture access is maintained it is very difficult to estimate intake and a tendency exists to underestimate overall feed/calorie intake. Restricting access to pasture can result in greater intake of grass over a short period34; therefore, even short periods of turnout can severely undermine programmes of weight loss.
When conditions are favourable, non-structural carbohydrate levels in grass can exceed 30%, comparable to levels in many cereal-based proprietary feeds. Sand or woodchip areas of turnout would be preferred as complete control over the diet is retained (Figure 6).

Hungry horses need to be monitored to ensure they do not ingest large quantities of bedding or other material, such as straw, hemp or wood shavings. Grazing muzzles can be an effective means of reducing pasture intake, although their effectiveness varies with design and between horses35,36; reductions in dry matter intake from 30% to 83% have been reported35-37.
Grazing muzzles must be used with care – an owner guide and video are available from the National Equine Welfare Council38. The authors recommend they should not be used 24/7 and, if used, should ideally be in place whenever turned out as part of the overall weight management programme, or rates of ingestion will increase at other times to compensate.
Hay should comprise the bulk of the ration in any obese horse or pony. Quality varies, and while forage analysis is cheap when performed through feed companies (approximately £30) and widely available, it is rarely performed in practice. The dry matter and nutrient content of hay varies, and will depend on the grass species present, time of cut and how the hay is cured.
When forage is fed at the levels required for weight loss, protein levels may already be insufficient19. Therefore, a low-calorie protein, vitamin and mineral balancer should always be included in the diet of animals offered restricted forage, and especially when feeding soaked hays.
A typical practical starting recommendation for a horse undertaking little or no exercise that requires more aggressive weight loss restriction would be to feed a mature grass-based hay at 1.5% BWT unsoaked (assuming 90% dry matter) or 1.8% if it is to be soaked (assuming 20% loss of dry matter with soaking) to promote weight loss (Figure 7).

Soaking is preferable as it allows a greater volume to be fed. It should be remembered the effects of soaking are variable, and any estimate is simply a starting point and will have to be adjusted according to the patient’s response.
Within any population managed in the same manner, large variation in body condition will exist between individuals and similar variation will occur in responses to dietary restriction. Some animals exhibit “weight loss resistance”, and may require repeated re-evaluation and further reduction in their feed intake19.
Feed intakes of 1.1% BWT of unsoaked hay – or 1.3% BWT of soaked hay – are considered the minimum desirable level43. In one study – in which hay produced commercially in the UK was soaked for 16 hours at 8°C – the reduction in WSC ranged from 6% to 54%, with a mean of 27%42. By contrast, in another study, soaking for 14.5 hours (± 2.1 hours) resulted in a mean reduction of 50.1% non-structural carbohydrate, with little variation between samples44.
Water temperature can have a marked effect – soaking in water of 16°C for 1 hour has a similar effect on sugar content as soaking at 8°C for 16 hours; in colder weather, the use of warm water should be encouraged39.
In addition to reducing WSC content, as aforementioned, soaking hay will also reduce the DM, soluble protein, vitamin and mineral content of hay40-42,45 – hence the need for a protein, vitamin and mineral balancer.
In horses and ponies, up to 30% of the hay ration can be replaced by straw (donkeys typically do well on 100% straw with an appropriate balancer), to reduce calorie intake further while maintaining fibre intake. Straw should be introduced gradually to help prevent gastrointestinal complications – and needs to be of high hygienic quality, with the seed heads shaken free before feeding.
If weight loss is not achieved having reduced feed to 1% of BWT as dry matter of low sugar (less than 10% to 12% WSC) forage – and no doubts exist over compliance – treatment with levothyroxine may be considered. Other energy-providing feedstuffs, such as cereals, and oils should be eliminated from the diet.
Exercise
Exercise increases insulin sensitivity, but the effect of exercise on ID in horses is inconsistent between studies. In some studies, relatively short periods of exercise (20 to 30 minutes) appear to increase insulin sensitivity, even in the absence of weight loss46, and overweight ponies that were exercised for six weeks while on a controlled diet had improved insulin sensitivity.
In one study, diet restriction with or without 15 minutes of brisk trotting (with a five-minute walk before and after) five days per week for 12 weeks resulted in the same amount of weight loss, but the exercised group also showed an improvement in its insulin sensitivity47.
Exercise is a not a substitute for an appropriate diet and moderate exercise in the absence of dietary restriction has no effect on weight loss48,49. Given the effects of exercise on insulin sensitivity are short-lived50, exercise – whenever clinically appropriate – should be regular in horses that have insulin dysregulation and should be continued after the desired level of weight loss has been achieved.
Rugging
A misconception exists among horse owners that horses require rugs in all but the finest weather. The use of rugs limits energy losses and they should, therefore, be avoided where possible.
Loss of weight during colder winter weather is normal and should be encouraged where horses have gained weight through the summer.
Medical treatments for obesity
Pharmaceuticals should not be an excuse for poor compliance with weight loss protocols.
Levothyroxine has been demonstrated to be an effective means of inducing weight loss and increasing insulin sensitivity in small numbers of normal horses51-53. The induction of a hyperthyroid state has been demonstrated to be safe in small numbers of horses54,55, but further, larger studies are required.
Levothyroxine administration has been reported as an adjunct to dietary management in horses that cannot be exercised56, and has been used in horses with “weight loss resistance” and in severe cases of laminitis associated with insulin dysregulation that were not responding despite conventional management.
Recommendations for the use of levothyroxine are not evidence-based, but derived from extensive clinical experience in the US where a three-month to six-month duration of treatment at a dosage of 0.1mg/kg orally once daily is recommended. Treatment is continued until target BWT has been reached – at which point the dose is decreased by 50% to 0.05mg/kg for two weeks, before being reduced by 50% again to 0.025mg/kg for a further two weeks prior to discontinuing treatment57.
Metformin is used in EMS, but its efficacy is disputed, as very little of it reaches the circulation in horses58,59 and its use does not appear to be associated with detectable improvements in insulin sensitivity60. Metformin would not be indicated for the management of obesity in the absence of laminitis and ID.
In non-obese horses, metformin has been demonstrated to impair glucose absorption and reduce the rise in insulin that occurs following ingestion of glucose61, which may be of benefit in reducing glucose absorption and in limiting postprandial hyperinsulinaemia, which is a factor in the development of laminitis. However, effects are likely to be limited to a few hours post-administration and no robust evidence exists of improved outcomes in treated animals.
Conclusion
Obesity represents a significant threat to equine welfare as a factor in multiple health problems, most notably laminitis.
Education is required to alter perceptions of what constitutes a healthy BCS and what should be fed to maintain a healthy body condition.
Dietary restriction and management are central to reducing obesity, but exercise, pharmaceuticals and reduced rugging may all be valuable adjuncts in certain cases.
- Some drugs are used under the cascade.
References
- Menzies-Gow NJ et al (2017). Prospective cohort study evaluating risk factors for the development of pasture-associated laminitis in the United Kingdom, Equine Vet J 49(3): 300-306.
- Dugdale AH et al (2011). Assessment of body fat in the pony: part I. Relationships between the anatomical distribution of adipose tissue, body composition and body condition, Equine Vet J 43(5): 552-561.
- Dugdale AH et al (2012). Body condition scoring as a predictor of body fat in horses and ponies, Vet J 194(2): 173-178.
- Robin CA et al (2015). Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores, Equine Vet J 47(2): 196-201.
- Menzies-Gow NJ et al (2010). Severity and outcome of equine pasture-associated laminitis managed in first opinion practice in the UK, Vet Rec 167(10): 364–369.
- Watson TD et al (1992). Equine hyperlipaemia in the United Kingdom: clinical features and blood biochemistry of 18 cases, Vet Rec 131(3): 48-51.
- Cymbaluk NF and Christison GI (1990). Environmental effects on thermoregulation and nutrition of horses, Vet Clin North Am Equine Pract 6(2): 355-372.
- Vick MM et al (2006). Obesity is associated with altered metabolic and reproductive activity in the mare: effects of metformin on insulin sensitivity and reproductive cyclicity, Reprod Fertil Dev 18(6): 609-617.
- Adams AA et al (2009). Effect of body condition, body weight and adiposity on inflammatory cytokine responses in old horses, Vet Immunol Immunopathol 127(3-4): 286-294.
- Robles M et al (2018). Maternal obesity increases insulin resistance, low-grade inflammation and osteochondrosis lesions in foals and yearlings until 18 months of age, PLOS One 13(1): e0190309.
- Buckley P et al (2013). Misbehaviour in Pony Club horses: incidence and risk factors, Equine Vet J 45(1): 9-14.
- Bailey SR et al (2008). Hypertension and insulin resistance in a mixed-breed population of ponies predisposed to laminitis, Am J Vet Res 69(1): 122-129.
- McGregor-Argo C (2009). Appraising the portly pony: body condition and adiposity, Vet J 179(2): 158-160.
- Kearns CF et al (2002). Fat-free mass is related to one-mile race performance in elite standardbred horses, Vet J 163(3): 260-266.
- Potter SJ et al (2017). Incidence of laminitis and survey of dietary and management practices in pleasure horses and ponies in south-eastern Australia, Aust Vet J 95(10): 370-374.
- Karikoski NP et al (2015). Pathology of natural cases of equine endocrinopathic laminitis associated with hyperinsulinemia, Vet Pathol 52(5): 945-956.
- Kohnke J (1992). Feeding and Nutrition: the Making of a Champion, Birubi Pacific, New South Wales.
- Henneke DR et al (1983). Relationship between condition score, physical measurements and body fat percentage in mares, Equine Vet J 15(4): 371-372.
- Dugdale AH et al (2010). Effect of dietary restriction on body condition, composition and welfare of overweight and obese pony mares, Equine Vet J 42(7): 600-610.
- Carter RA et al (2009). Apparent adiposity assessed by standardised scoring systems and morphometric measurements in horses and ponies, Vet J 179(2): 204-210.
- Morrison PK et al (2014). Preliminary investigation into a potential role for myostatin and its receptor (ActRIIB) in lean and obese horses and ponies, PLOS One 9(11): e112621.
- Znamirowska A (2005). Prediction of horse carcass composition using linear measurements, Meat Sci 69(3): 567-570.
- Frank N et al (2006). Physical characteristics, blood hormone concentrations, and plasma lipid concentrations in obese horses with insulin resistance, J Am Vet Med Assoc 228(9): 1,383-1,390.
- Dugdale AH et al (2011). Effects of season and body condition on appetite, body mass and body composition in ad libitum fed pony mares, Vet J 190(3): 329-337.
- Giles SL et al (2015). Assessing the seasonal prevalence and risk factors for nuchal crest adiposity in domestic horses and ponies using the Cresty Neck Score, BMC Vet Res 11: 13.
- Argo CM et al (2012). Weight loss resistance: a further consideration for the nutritional management of obese Equidae, Vet J 194(2): 179-188.
- Bamford NJ et al (2016). Effect of increased adiposity on insulin sensitivity and adipokine concentrations in different equine breeds adapted to cereal-rich or fat-rich meals, Vet J 214: 14-20.
- Schultz N et al (2014). Factors associated with leptin and adiponectin concentrations in a large across breed cohort of horses and ponies, J Vet Intern Med 28(3): 1,114.
- Wray H et al (2013). Plasma concentrations of inflammatory markers in previously laminitic ponies, Equine Vet J 45(5): 546-551.
- Menzies-Gow NJ et al (2019). Validity and application of immunoturbidimetric and enzyme-linked immunosorbent assays for the measurement of adiponectin concentration in ponies, Equine Vet J 51(1): 33-37.
- Jocelyn NA et al (2018). Effect of varying the dose of corn syrup on the insulin and glucose response to the oral sugar test, Equine Vet J 50(6): 836-841.
- Morgan RA et al (2017). Carbonyl reductase 1 catalyzes 20β-reduction of glucocorticoids, modulating receptor activation and metabolic complications of obesity, Scientific Reports 7(1): 10633.
- Schott H et al (2017). Recommendations for the diagnosis and treatment of pituitary pars intermedia dysfunction (PPID), http://bit.ly/2wq8oPU
- Ince JC et al (2011). Changes in proportions of dry matter intakes by ponies with access to pasture and haylage for 3 and 20 hours per day respectively, for six weeks, J Equine Vet Sci 31(5): 283.
- Longland AC et al (2016). Effects of grazing muzzles on intakes of dry matter and water-soluble carbohydrates by ponies grazing spring, summer, and autumn swards, as well as autumn swards of different heights, J Equine Vet Sci 40: 26-33.
- Longland AC et al (2016). Efficacy of wearing grazing muzzles for 10 hours per day on controlling bodyweight in pastured ponies, J Equine Vet Sci 45: 22–27.
- Glunk EC et al (2014). Interaction of grazing muzzle use and grass species on forage intake of horses, J Equine Vet Sci 34(7): 930-933.
- National Equine Welfare Council (2015). Grazing muzzles, http://bit.ly/31WzFH8
- Longland AC et al (2014). Effect of period, water temperature and agitation on loss of water-soluble carbohydrates and protein from grass hay: implications for equine feeding management, Vet Rec 174(3): 68.
- Moore-Colyer MJS et al (2014). The effect of five different wetting treatments on the nutrient content and microbial concentration in hay for horses, PLOS One 9(11): e114079.
- Argo CM et al (2015). Considerations for the use of restricted, soaked grass hay diets to promote weight loss in the management of equine metabolic syndrome and obesity, Vet J 206(2): 170-177.
- Longland AC et al (2011). Effects of soaking on the water-soluble carbohydrate and crude protein content of hay, Vet Rec 168(23): 618.
- Harris PA et al (2017). Review: feeding conserved forage to horses: recent advances and recommendations, Animal 11(6): 958-967.
- Mack SJ et al (2014). Impact of water-soaking on the nutrient composition of UK hays, Vet Rec 174(18): 452.
- McGowan CM et al (2013). Dietary restriction in combination with a nutraceutical supplement for the management of equine metabolic syndrome in horses, Vet J 196(2): 153-159.
- Powell DM et al (2002). Effect of short-term exercise training on insulin sensitivity in obese and lean mares, Equine Vet J 34(S34): 81-84.
- Bamford NJ et al (2019). Influence of dietary restriction and low-intensity exercise on weight loss and insulin sensitivity in obese equids, J Vet Intern Med 33(1): 280-286.
- Carter RA et al (2010). Effects of exercise training on adiposity, insulin sensitivity, and plasma hormone and lipid concentrations in overweight or obese, insulin-resistant horses, Am J Vet Res 71(3): 314-321.
- Turner SP et al (2011). Comparison of insulin sensitivity of horses adapted to different exercise intensities, J Equine Vet Sci 31(11): 645-649.
- de Graaf-Roelfsema E et al (2012). Effects of intensified training and subsequent reduced training on glucose metabolism rate and peripheral insulin sensitivity in Standardbreds, Am J Vet Res 73(9): 1,386-1,393.
- Frank N et al (2005). Effects of oral administration of levothyroxine sodium on concentrations of plasma lipids, concentration and composition of very-low-density lipoproteins, and glucose dynamics in healthy adult mares, Am J Vet Res 66(6): 1,032-1,038.
- Tóth F et al (2010). Effects of pretreatment with dexamethasone or levothyroxine sodium on endotoxin-induced alterations in glucose and insulin dynamics in horses, Am J Vet Res 71(1): 60-68.
- Frank N et al (2008). Effects of long-term oral administration of levothyroxine sodium on glucose dynamics in healthy adult horses, Am J Vet Res 69(1): 76-81.
- Sommardahl CS et al (2005). Effects of oral administration of levothyroxine sodium on serum concentrations of thyroid gland hormones and responses to injections of thyrotropin-releasing hormone in healthy adult mares, Am J Vet Res 66(6): 1,025-1,031.
- Frank N et al (2008). Effects of long-term oral administration of levothyroxine sodium on serum thyroid hormone concentrations, clinicopathologic variables, and echocardiographic measurements in healthy adult horses, Am J Vet Res 69(1): 68-75.
- Durham AE (2017). Therapeutics for equine endocrine disorders, Vet Clin North Am Equine Pract 33(1): 127-139.
- Frank N (2006). Insulin resistance in horses, Proceedings of the 52nd Annual Convention of the American Association of Equine Practitioners, San Antonio.
- Tinworth KD et al (2010). Pharmacokinetics of metformin after enteral administration in insulin-resistant ponies, Am J Vet Res 71(10): 1,201-1,206.
- Hustace JL et al (2009). Pharmacokinetics and bioavailability of metformin in horses, Am J Vet Res 70(5): 665-668.
- Tinworth KD et al (2012). The effect of oral metformin on insulin sensitivity in insulin-resistant ponies, Vet J 191(1): 79-84.
- Rendle DI et al (2013). Effects of metformin hydrochloride on blood glucose and insulin responses to oral dextrose in horses, Equine Vet J 45(6): 751-754.
