12 Jan 2021
Adele Williams covers considerations for equine parasite control, taking into account what is currently known about anthelmintic resistance in UK equine parasites.
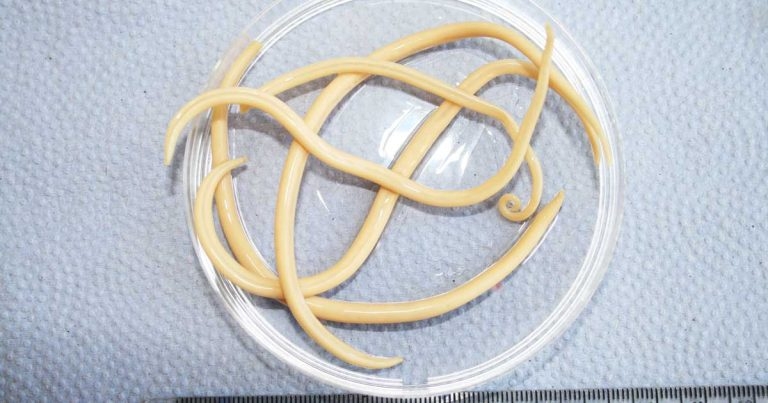

Anthelmintic resistance is recognised to be commonplace in UK equine endoparasites, and the situation is getting worse (Rendle et al, 2019; Tzelos and Matthews, 2016).
As guardians of equine health, we have to strive to get the balance right between limiting parasitism that causes clinical disease, while minimising propagation of anthelmintic resistance. Unlike in small animals, prophylactic use of anthelmintics in horses should be avoided.
Horse owners have access to equine anthelmintics without the need for veterinary prescription, and blanket deworming of all horses has been standard practice for many decades. This has meant horse owners have the mindset of needing to worm every animal on their premises, and the ability to do this with free access to the anthelmintics at their disposal. The non-POM-V classification of anthelmintics also means veterinary advice is infrequently sought for routine endoparasite control (Easton et al, 2016).
We, as vets, know targeted deworming strategies and parasite control are needed, and that refugia (the pool of anthelmintic sensitive parasites in the environment) need to be protected to keep the resistance genes diluted (Kaplan and Nielsen, 2010; Nielsen et al, 2014; Peregrine et al, 2014).
We have been encouraging owners to have faecal worm egg counts performed and treat only those that need treating. However, the evidence, both published and anecdotal across the UK, has indicated that anthelmintic resistance prevalence continues to increase, which would suggest our advice is not being heeded (Rendle et al, 2019).
While it is true some horse owners have now embraced faecal testing and targeted deworming of those with high faecal worm egg counts (FWECs), this measure alone is not proving sufficient to curb resistance. Owners who have FWECs performed do not necessarily understand the results or adhere to advice of the animals to treat, or with which anthelmintic. Testing of anthelmintic effectiveness is not widely performed by UK horse owners, so little understanding exists of efficacy or comprehension of the relevance of anthelmintic resistance to their horses (Rendle et al, 2019).
A huge effort in client education is required if we are to stand a chance of slowing the upward curve of anthelmintic resistance. No new anthelmintics are available for use in horses; we must preserve those we have.
Every vet who does any amount of equine work must strive to encourage best practice endoparasite control measures, so we can achieve that delicate balance of minimising disease while preventing anthelmintic resistance. Much has advanced in equine parasite control strategies in the past few years, and it is a complex subject; therefore, we must make sure we, as equine vets, are current on our own knowledge before disseminating it to clients.
All equids should be assessed for the need for anthelmintic treatment before any is administered. Over time, testing saves money compared with blind treatment protocols (Lester et al, 2013a). A combination of tests should be implemented to help assess endoparasite burden at different times throughout the year.
Anthelmintic resistance can be gauged by performance of faecal egg count reduction tests (FECRTs; Lester and Matthews, 2014). To perform a FECRT, a FWEC should be performed prior to – and 10 to 14 days following – anthelmintic treatment. Anthelmintic data sheets have published expected percentages of egg reduction following treatment. If the percentage egg reduction achieved is less than that published on the data sheet, then either treatment has failed through underdosing or product spillage, or resistance is present. To confirm resistance, the FECRT needs to be assessed on the entire herd of horses that have high initial FWEC warranting anthelmintic treatment (ideally more than six animals), rather than an individual animal, which may have been underdosed through weight underestimation or had not received the entire intended dose. The FECRT can be calculated by assessing quantitative FWEC performed before and 10 to 14 days post-anthelmintic treatment. The FECRT based on mean FWEC of all treated animals before (day 0) and after treatment (days 10 to 14) can be calculated as follows:
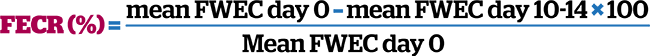
Early resistance can also be indicated by assessing efficacy of a product following treatment with its data sheet expected FECR percentage and expected faecal egg reappearance period (ERP). Table 1 summarises data regarding anthelmintic resistance for common equine endoparasites in UK horse populations.
| Table 1. Anthelmintic resistance in UK common equine endoparasites based on published and anecdotal reports. Strongyle resistance assessed by faecal egg count reduction test. Lowered sensitivity to drug indicated by reduced egg reappearance period compared to product data sheet expected egg reappearance times (“–” where anthelmintic not indicated in that species). | |||||
|---|---|---|---|---|---|
| Fenbendazole | Moxidectin | Ivermectin | Pyrantel | Praziquantel | |
| Cyathastomins | Universal resistance (single and 5-day treatments) | Lowered sensitivity to drug common | Low level of resistance, lowered sensitivity to drug common | 0-70% resistance | – |
| Ascarids (Parascaris equorum) | Some resistance on stud farms | Resistance common on stud farms | Moderate resistance, common to universal on stud farms | Some resistance | – |
| Tapeworms (Anoplocephala perfoliata) | – | – | – | Resistance suspected | Resistance suspected |
| Content based on Lester et al (2013b), Molena et al (2018), Relf et al (2014), Rendle et al (2019), Stratford et al (2014) and Tzelos et al, (2017). | |||||
If resistance is identified, a different class of anthelmintic should be used, with repeat FECRT performed to assess resistance to that drug. Not only that, but assessment of the environment, stocking density, pasture management and higher risk animals should be undertaken, with steps implemented to minimise further increases in resistance.
If resistance is identified, it is really important to work with the owner to identify areas where practical action can take place to help fight the development and perpetuation of anthelmintic resistance. This includes not only anthelmintic-driven measures, but also non-anthelmintic management measures (see later in this article).
Our patients need protecting from parasite-related disease and, at the same time, the parasites must be susceptible to the anthelmintics used, so we mustn’t overuse anthelmintics driving resistance to them. Judicial choice of which animals are at high risk and require treating – and appropriate treatment choice, along with protection of refugia and environmental management to limit parasite environmental load – is necessary to avoid selection of resistant parasites.
Different age groups are at different risk of disease, with foals and young animals being more susceptible and more likely to be higher shedders (Relf et al, 2013). Aged animals above 15 years old are at a moderately increased risk of parasite susceptibility and shedding. Healthy adult horses younger than 15 years old are usually lowest risk.
Horses’ risk category can be further assessed by considering the paddock stocking density, the frequency of faecal removal from pasture and herd stability (closed versus open; Rendle et al, 2019). Any premises with youngstock will be at higher risk and animals should have FWEC performed more regularly. The less frequently faeces is removed, the higher the risk (Tzelos et al, 2017). Closed herds are more protected than those with new animals being regularly introduced.
The FWEC value attributable to warranting an anthelmintic routine treatment during the grazing season varies depending on the risk group of the horse.
As a general rule, treatment should be considered for those horses with FWEC more than 200eggs/g to 250eggs/g, but can be as high as more than 500 eggs/g in low-risk horses on low stocking density, well-managed pastures in a stable herd (Rendle et al, 2019).
Some adult animals within a herd will be more susceptible to parasites than others, so that a small proportion of the population is responsible for a large proportion of parasite dissemination (Lester et al, 2013b). Multiple FWECs should be used to assess an individual adult animal’s worm burden over time, so it can be classified as a repeat offender or lower risk. Targeted treatment of individuals with high burdens will help to reduce pasture contamination, increase intervals needed between treatments and protect refugia (Rendle et al, 2019).
Underdosing due to poor estimation of weight is an often overlooked factor in failure of anthelmintic treatment. Owners and vets alike should not guess weight by appearance only, as this leads to inaccurate dosing. You must insist that each individual animal has its weight calculated as accurately as possible to avoid underdosing, and also to protect the animal from overdosing and potential toxic side effects.
The gold standard for this is use of a weighbridge, although that will not be available to most. The least that should be done is use of a weigh tape. If the owner doesn’t have a weigh tape, weight can easily be estimated using tape measure measurement (in inches) of heart girth and body length (from point of shoulder to point of buttocks) using the following formula:

For weight in kilos, the calculated answer can be divided by 2.2.
The other area owners require coaching on to avoid treatment failure is effective dosing and avoiding spillage of product. Only the anthelmintic that is swallowed will be effective, and getting only half in will lead to underdosing and add to anthelmintic resistance. This can be the most challenging aspect of parasite control in some horses, and you may need to spend some time with the owner to help him or her get this right.
The owner may need to train the horse to accept oral medication (for example, using clicker training), or otherwise disguise the medication in a Trojan treat (such as a jam sandwich).
Moxidectin should be reserved for larvicidal treatment of cyathostomes in high-risk animals and animals with clinical cyathostomiasis, since it is now the only effective drug in the UK. Fenbendazole resistance is universally common, in cyathostomes at least, so should be avoided for most treatments.
The only occasion when fenbendazole may be considered is for treatment of heavy ascarid burdens in foals and yearlings (Rendle et al, 2019). Ivermectin and pyrantel should, therefore, be the routine drugs of choice for routine anthelmintic treatment of horses with high FWEC. Pyrantel and praziquantel are both effective for treatment of tapeworms.
If we reduce the need for anthelmintic treatment, by reducing environmental infection levels, then fewer horses will need treatment in the first place. Environmental management practices are often taken as presumed, but are worth reiterating and reinforcing to keep horses safe from both disease and anthelmintic resistance.