1 Jun 2021
Equine respiratory diseases part 2: non-infectious
Sarah Gough – in her final article in this series – looks at the types, causes, treatment and management of non-infectious disease.


Equids have a huge respiratory capacity that enables them to perform high-intensity exercise such as racing, and one-day and three-day eventing.
During these elite sports they call on their respiratory reserves to enable that peak level of performance. As such, any condition that compromises respiratory function can result in reduced performance in these elite athletes and, likewise, the respiratory reserve can mask mild disease in horses that are not performing high-intensity exercise, as a mildly compromised respiratory function may still provide sufficient oxygen.
Respiratory diseases is an extensive topic that cannot be covered in a single article. As such, this review follows the review on some of the infectious respiratory diseases in part one and focuses on non-infectious causes of respiratory disease.
These non-infectious diseases, such as equine asthma (EA) and exercise-induced pulmonary haemorrhage (EIPH), result in reduced exercise capacity that may be subclinical or clinical in nature, and varying degrees of systemic compromise depending on severity.
EA
EA is a common, non-infectious respiratory disease that encompasses a spectrum of chronic allergic airway inflammation that is variable in its clinical presentation, severity and reversibility.
Three primary clinical presentations for EA exist:
- mild EA typically presents as a horse with “poor” or reduced performance in the absence of clinical signs that correlate the reduction in performance to the respiratory tract
- moderate EA typically presents as a horse with mild clinical signs of respiratory dysfunction during exercise
- severe EA presents as a horse with clinical signs of respiratory dysfunction at rest as well as during exercise1
Both mild and moderate EA were formerly classified as inflammatory airway disease, while severe EA was formerly assigned recurrent airway obstruction (RAO) or summer pasture-associated RAO depending on the triggering factors.
Irrespective of the severity of disease, affected horses have altered respiratory function, reduced performance or, in some cases, exercise intolerance and an altered bronchoalveolar lavage fluid (BALF) leukocyte profile, secondary to chronic lower airway inflammatory changes.
Cause
While it is widely accepted that EA is an immune-mediated inflammatory response to inhaled aeroallergens, the underlying trigger to developing inflammation remains incompletely understood. An extensive discussion on the pathophysiology of EA is beyond the scope of this publication – readers are directed to the spring 2021 Vet Times Equine supplement (VTE7.1) in which EA was discussed in more depth.
However, to summarise the cause of EA, we know that organic material within the respirable zone of stables and dusty environments effectively induces airway neutrophilia, which, although an important part of the immune response and airway defence mechanisms, can be proinflammatory within the airways when dysregulated2.
In addition, both metachromatic and eosinophilic phenotypes may be seen. While not definitive, an association seems to exist between airway hyper-reactivity and airway obstruction for metachromatic cases, compared with tracheal mucous accumulation and cough associated with neutrophilic inflammation. Likewise, eosinophilic asthma seems more common in young horses associated with exposure to dust3.
Once the cascade of inflammation is stimulated, the disease process, which features components of both type-one and type‑three hypersensitivities, results in airway hyper-responsiveness, exercise-induced hypoxaemia secondary to impaired gas exchange and lower airway obstruction in mild-moderate cases2,3.
In addition, horses with severe EA experience airway remodelling with chronicity, secondary to the bronchoconstriction and bronchiolitis that develops, as well as mucous secretion and accumulation in the lower airways1,4. Over time, as the airway remodelling progresses, if not managed, fibrosis and reduced airway compliance of both peripheral and central airways ensues1.
While currently unproven in horses, viral respiratory diseases have been shown to act as triggers for asthma in human patients. Initial studies in horses have shown an association between EA, and equine rhinitis A virus and equine herpesvirus-2 nasal shedding5.
In theory, infection with these viruses typically results in airway neutrophilia and mucous production, which, as described previously, may precipitate a dysregulated inflammatory state. However, as these viruses circulate regularly among equine patients this association does not equate to causation, although it does warrant further investigation.
Diagnosis
Diagnosis of EA requires recognition of the early clinical signs, lower airway sampling for cytological evaluation and response to bronchodilation in severe cases1. This necessitates a good clinical history and application of the 23-point modified clinical score to differentiate the “grades” of EA into mild, moderate and severe.
Despite reluctance among some practitioners, cytological evaluation of BALF remains superior to cytological evaluation of tracheal wash (TW) samples with respect to both the sensitivity and specificity of diagnosing EA6‑9. However, a recent study showed that when evaluating neutrophilic inflammation there was substantial agreement between TW and BALF cytological profiles, although TW cytology was less reliable for diagnosis of non-neutrophilic phenotypes, such as metachromatic and eosinophilic asthma10. The authors of that study suggested that in their study population a TW was a comparable diagnostic tool for diagnosis of severe EA.
Individual practitioners have varying approaches to performing a bronchoalveolar lavage (BAL) with typical infusion volumes between 250ml to 500ml. Assuming an infusion volume around 250ml, appropriate cut-off values for mild-moderate EA is greater than 10% neutrophils, greater than 5% mast cells and/or greater than 5% eosinophils, and in horses with severe EA a neutrophil count greater than 25% may be expected, except during periods of remission3,6.
Treatment
Despite extensive research and a steady increase in our understanding of the pathophysiology of EA, the treatment and management of affected horses remains challenging. Successful management of EA almost always requires lifelong environmental management in conjunction with short-term therapeutic interventions during periods of disease exacerbations. In rare cases of mild-moderate EA, environmental management alone may be sufficient.
The mainstay of therapeutic intervention for EA includes administration of corticosteroids with or without concurrent use of bronchodilators. While initially systemic administration of corticosteroids such as prednisolone or dexamethasone is often necessary in cases of severe EA7,11, it is advisable to transition to inhaled corticosteroids as soon as possible12,13 to reduce both the immunosuppressive effects and the anecdotal risk of laminitis associated with systemic administration of corticosteroids.
According to a meta-analysis performed in 2018, both systemically administered and inhaled corticosteroids effectively reduced clinical signs of EA; however, the rate of response was more rapid with systemic administration compared with inhaled medications11.
The Aservo EquiHaler is a relatively new product that uses a pro-drug delivery system, which, on contact with the respiratory epithelium, is converted into the active drug des‑ciclesonide, a potent corticosteroid, with minimal systemic absorption. While it seems anecdotally that this product is relatively effective in the management of EA, multiple courses are often necessary.
Concurrent use of bronchodilators may help to facilitate delivery of inhaled corticosteroid to the distal airways, as well as to relieve bronchospasm and bronchoconstriction, enabling clearance of mucous plugs to improve lower airway ventilation. In addition, during acute exacerbations, bronchodilators such as N-butylscopolamine (Buscopan) and atropine play an important role as rescue therapies, with effects seen within five minutes of parenteral administration7,14.
Bronchodilators such as salbutamol and clenbuterol may be beneficial for two to four weeks’ treatment alongside corticosteroid treatment, although it is important to note that response to treatment with sole therapy often becomes refractory15, hence the importance of concurrent corticosteroid treatment and environmental management.
Although not yet widely available and still in its infancy in terms of clinical trials, the use of immunomodulatory inhalational therapies to reduce airway inflammation through regulatory T cell activation and restoration of T helper cell balance may be a viable treatment option in the future, pending further work on these therapies16.
EIPH
EIPH is a common occurrence in both Thoroughbred and standardbred racing horses, as well as in horses in other high-intensity performance disciplines, and is associated with reduced athletic performance. Up to 55% of racehorses have tracheobronchoscopic evidence of EIPH17,18 and according to the American College of Veterinary Internal Medicine consensus statement, EIPH occurs in “the majority of Thoroughbred and standardbred racehorses”19.
Similarly, it has been shown that the EIPH prevalence increases with increasing endoscopic examinations18, suggesting that episodes may be intermittent in nature. However, despite its common occurrence it is important to consider EIPH as a disease that requires management, and not an expected manifestation of strenuous exercise.
The effects of EIPH on respiratory physiology have not been convincingly determined; however, the consensus statement concluded that some evidence exists that EIPH has an adverse effect on arterial oxygen tension and induces an increase in blood lactate concentration19.
The effects of EIPH on performance have been somewhat variable20. A study in Thoroughbred racehorses showed that horses with grade 0-1 EIPH (none to mild EIPH) had 4 times more likelihood of winning, 1.8 times more likelihood of placing (positions 1-3) and were 3 times more likely to have race earnings in the 90th percentile when compared with horses that experienced grade 2-4 EIPH (moderate to severe EIPH)21.
Other studies have shown that horses with more severe EIPH (grades 3-4) were more likely to have reduced race-day performance, finish further from the winner and while as a group they were significantly faster in the early-mid stages of the race, they were slower in the final stages, likely due to capillary stress failure and EIPH18.
This early speed was also shown in a study of barrel racing horses, in which those with severe EIPH were significantly faster than horses without EIPH22, as well as in treadmill studies suggesting that rapid acceleration may induce higher pulmonary artery pressures than a steady increase in speed.
While not life-threatening in the majority of cases, some evidence exists that severe pulmonary haemorrhage is at least contributory in 18% to 60% of sudden deaths not related to catastrophic musculoskeletal failure during racing23,24. In addition, some evidence exists that tracheobronchoscopically evident EIPH is both progressive and irreversible18, hence the importance of early management of this condition.
Cause
Although not yet completely understood, the most universally accepted theory as to the cause of EIPH is pulmonary capillary stress failure as a result of the combination of markedly high intravascular pressure and low airway pressure that occurs during high‑intensity exercise20. Both histological and gross evaluation of pulmonary tissue shows bilateral lesions distributed primarily in the caudodorsal lung fields19.
If we look to postmortem studies on EIPH-affected horses, the lesions are characterised grossly by pleural discolouration with haemosiderin (from chronic haemorrhage), and histologically by fibrosis of adjacent alveolar and interlobular septa, as well as the pleura itself25. In addition, vasculogenesis often exists, as well as remodelling of small pulmonary veins. The latter results in adventitial collagen deposition, as well as smooth muscle hyperplasia25.
Such vascular remodelling is typically secondary to increased venous pressure or endothelial stress, and in racing horses is thought to be secondary to the extreme flow and pressures that occur during racing. This is further supported by the typical caudodorsal distribution as this region of the lungs receives the greatest flow.
The corollary of vascular thickening is a reduction in compliance, which reduces the vessels’ ability to withstand the increases in blood flow during exercise and, hence, results in physiological obstruction. A vicious cycle then develops, with obstruction downstream resulting in increasing capillary pressures upstream and a propensity for stress failure25. It is this capillary stress failure, rather than the remodelling itself, that results in EIPH.
Diagnosis
The gold standard for diagnosis of EIPH is based on tracheobronchoscopic visualisation of blood post-exercise and/or cytological evidence of red blood cells in BALF collected post-exercise. An endoscopic grading system has been developed, and is shown in Table 1 and Figure 1.
| Table 1. Tracheobronchoscopic grading system described for exercise-induced pulmonary haemorrhage (EIPH) based on the scoring system proposed by Hinchcliff et al26 | |
|---|---|
| EIPH grade | Tracheobronchoscopic findings |
| Grade 0 | No blood observed. |
| Grade 1 | ≥1 blood speck or ≤2 short (<1/4 tracheal length) and narrow (<10% tracheal surface area) streams of blood. |
| Grade 2 | 1 stream of blood that goes at least 1/2 tracheal length or >2 short streams >1/3 tracheal circumference. |
| Grade 3 | Multiple distinct streams covering >1/3 tracheal circumference without pooling at the thoracic inlet. |
| Grade 4 | Multiple, coalescing streams cover >90% tracheal surface with blood pooling at the thoracic inlet. |
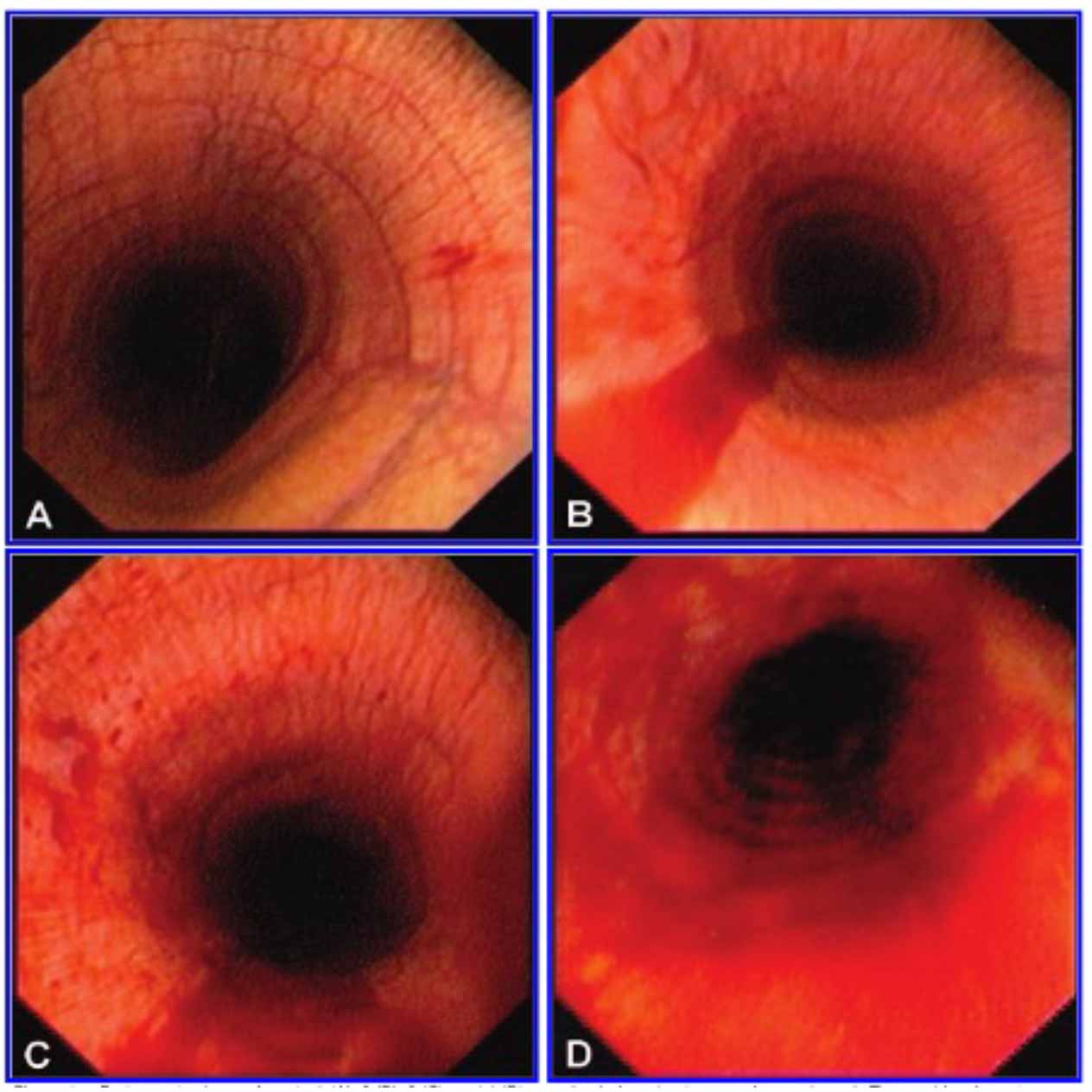
While the detection of blood on tracheobronchoscopy has a high specificity for EIPH, the sensitivity is lower as the presence of blood in the trachea can be influenced by the severity of lower airway haemorrhage, time elapsed between exercise and examination (both too soon or too long) and an inappropriate level of exercise20.
The use of both lower airway cytology (BALF cytology) and tracheobronchoscopic examination arguably provides the highest sensitivity and specificity. As with tracheobronchoscopic examination, the sensitivity and specificity of cytological examination of BALF is also not 100%.
Confounding factors with this technique include a variable persistence of haemosiderophages in the lower airway after lower airway haemorrhage, a non-linear relationship between the severity of EIPH and the number of haemosiderophages present, and the variable distribution of haemorrhage in the lower airways. As BAL tubes are generally passed blindly, the exact location of sampling is not known, although is typically the caudodorsal lung lobe20.
Additional diagnostic tools that may aid in the diagnosis include radiographic evaluation, which may show radiodensity in the caudodorsal lung fields, and clinical examination, which may identify epistaxis, increased respiratory rate, and/or effort and coughing. However, it is important to note that none of the above are pathognomonic for EIPH; many horses with EIPH do not show any radiographic or ultrasonographic abnormalities, and both coughing and epistaxis are variable findings that can also be associated with other conditions19.
While epistaxis is commonly considered to go hand in hand with EIPH, it is important to remember that epistaxis is only a marker for the most severe form and, in fact, many affected horses do not display any signs of blood at the nares, hence the importance of tracheobronchoscopy immediately post-exercise, and/or gross and cytological examination of BALF.
Treatment
Despite several studies with conflicting results, the current consensus statement concluded that high-quality evidence exists that furosemide reduced both the incidence of EIPH and severity of EIPH19.
In the author’s experience, the use of furosemide (0.25mg/kg to 0.5mg/kg IV) four hours prior to fast work helps to reduce the incidence of EIPH, although it is important to adhere to local performance society jurisdiction withholding periods. In regions where race day administration is permissible, this may also be beneficial in horses that experience EIPH.
Additional interventions – including the use of aminocaproic acid, NSAIDs, corticosteroids, bronchodilators and pentoxifylline – were concluded to have low-quality evidence supporting their efficacy for reducing the severity of EIPH in the consensus statement19.
Pharmacological interventions, although arguably the most effective means of managing EIPH, are not permitted in many racing and high‑intensity performance jurisdictions, and, as such, other management strategies need to be employed.
Many risk factors have been identified, including cold weather, race distance (albeit with conflicting evidence between “staying distance” and “sprint distance” being higher risk), number of starts in a career and number of days in any one preparation, with none conclusively established in all cases. However, in horses with confirmed EIPH, avoidance of racing and training during winter may be beneficial in reducing disease progression and severity.
In addition, encouraging a steady increase in speed, particularly during training, may help to reduce the incidence of EIPH in those horses during training. Likewise, a settled midfield position during the early part of the race may help to protect against EIPH in horses that tend to have early speed or rapid acceleration.
Summary
Respiratory diseases are common in equine patients and vary in severity, from subclinical disease to severe compromise and even death. Regardless of the disease, early detection and intervention is important to reduce the severity and progression of disease for non-infectious diseases.
Timely and accurate diagnosis of respiratory diseases requires accurate evaluation of clinical signs, consideration of patient signalment and history, and correct sampling techniques and sample handling based on the temporal aspects of each individual case.
- Some drugs are used under the cascade.
References
- Couëtil LL, Cardwell JM, Léguillette R, Mazan M, Richard EA, Bienzle D, Bullone M, Gerber V, Ivester K, Lavoie J-P, Martin J, Moran G, Niedźwiedź A, Pusterla N and Swiderski C (2020). Equine asthma: current understanding and future directions, Frontiers in Veterinary Science 7: 450.
- Uberti B and Morán G (2018). Role of neutrophils in equine asthma, Animal Health Research Reviews 19(1): 65-73.
- Couëtil LL, Cardwell JM, Gerber V, Lavoie J-P, Léguillette R and Richard EA (2016). Inflammatory airway disease of horses – revised consensus statement, Journal of Veterinary Internal Medicine 30(2): 503-515.
- Barton AK and Gehlen H (2016). Pulmonary remodeling in equine asthma: what do we know about mediators of inflammation in the horse? Mediators of Inflammation, 2016: 5693205.
- Couëtil LL, Hoffman AM, Hodgson J, Buechner-Maxwell V, Viel L, Wood JLN and Lavoie J-P (2007). Inflammatory airway disease of horses, Journal of Veterinary Internal Medicine 21(2): 356-361.
- Cian F, Monti P and Durham A (2015). Cytology of the lower respiratory tract in horses: an updated review, Equine Veterinary Education 27(10): 544-553.
- Mazan MR (2018). Lower airway disease in the athletic horse, Veterinary Clinics of North America: Equine Practice 34(2): 443-460.
- Richard EA and Robinson NE (2016). Inflammatory Airway Disease Congress: one syndrome, multiple pathways: a Dorothy Russell Havemeyer symposium, Equine Veterinary Education 28(1): 9-12.
- Hewson J and Arroyo LG (2015). Respiratory disease: diagnostic approaches in the horse, Veterinary Clinics of North America: Equine Practice 31(2): 307-336.
- Rossi H, Virtala AM, Raekallio M, Rahkonen E, Rajamäki MM and Mykkänen A (2018). Comparison of tracheal wash and bronchoalveolar lavage cytology in 154 horses with and without respiratory signs in a referral hospital over 2009-2015, Frontiers in Veterinary Science 5: 61.
- Calzetta L, Rogliani P, Page C, Roncada P, Pistocchini E, Soggiu A, Piras C, Urbani A and Matera MG (2018). Clinical effect of corticosteroids in asthma-affected horses: a quantitative synthesis, Equine Veterinary Journal 50(5): 594-601.
- Haspel AD, Giguère S, Hart KA , Berghaus LJ and Davis JL (2018). Bioavailability and tolerability of nebulised dexamethasone sodium phosphate in adult horses, Equine Veterinary Journal 50(1): 85-90.
- Mainguy-Seers S, Picotte K and Lavoie J-P (2018). Efficacy of tamoxifen for the treatment of severe equine asthma, Journal of Veterinary Internal Medicine 32(5): 1,748-1,753.
- Arroyo MG, Couëtil LL, Nogradi N, Kamarudin MM and Ivester KM (2016). Efficacy of inhaled levalbuterol compared to albuterol in horses with recurrent airway obstruction, Journal of Veterinary Internal Medicine 30(4): 1,333-1,337.
- Calzetta L, Crupi R, Roncada P, Pistocchini E , Cave D, Rossi I, Cito G, Jacobson GA and Britti D (2020). Clinical efficacy of bronchodilators in equine asthma: looking for minimal important difference, Equine Veterinary Journal 52(2): 305-313.
- Klier J, Bartl C, Geuder S, Geh KJ, Reese S, Goehring LS, Winter G and Gehlen H (2019). Immunomodulatory asthma therapy in the equine animal model: a dose-response study and evaluation of a long‑term effect, Immunity, Inflammation and Disease 7(3): 130-149.
- Preston S and Riggs CM (2015). Descriptive analysis of longitudinal endoscopy for exercise-induced pulmonary haemorrhage in Thoroughbred racehorses training and racing at the Hong Kong Jockey Club – letter, Equine Veterinary Journal 47(3): 374-375.
- Crispe EJ, Secombe CJ, Perera DI, Manderson AA, Turlach BA and Lester GD (2019). Exercise-induced pulmonary haemorrhage in Thoroughbred racehorses: a longitudinal study, Equine Veterinary Journal 51(1): 45-51.
- Hinchcliff KW, Couetil LL, Knight PK, Morley PS, Robinson NE, Sweeney CR and van Erck E (2015). Exercise induced pulmonary hemorrhage in horses: American College of Veterinary Internal Medicine consensus statement, Journal of Veterinary Internal Medicine 29(3): 743-758.
- Crispe EJ and Lester GD (2019). Exercise-induced pulmonary hemorrhage: is it important and can it be prevented? Veterinary Clinics of North America: Equine Practice 35(2): 339-350.
- Hinchcliff KW, Jackson MA, Morley PS, Brown JA, Dredge AE, O’Callaghan PA, McCaffrey JP, Slocombe RE and Clarke AE (2005). Association between exercise‑induced pulmonary hemorrhage and performance in Thoroughbred racehorses, Journal of the American Veterinary Medical Association 227(5): 768-774.
- Léguillette R, Steinmann M, Bond SL and Stanton B (2016). Tracheobronchoscopic assessment of exercise-induced pulmonary hemorrhage and airway inflammation in barrel racing horses, Journal of Veterinary Internal Medicine 30(4): 1,327-1,332.
- Boden L, Charles JA, Slocombe RF, Sandy JR, Finnin PJ, Morton JM and Clarke AF (2005). Sudden death in racing Thoroughbreds in Victoria, Australia, Equine Veterinary Journal 37(3): 269-271.
- Lyle C, Uzal FA, McGorum BC, Aida H, Blissitt KJ, Case JT, Charles JT, Gardner I, Horadagoda N, Kusano K, Lam K, Pack JD, Parkin TD, Slocombe RF, Stewart BD and Boden LA (2011). Sudden death in racing Thoroughbred horses: an international multicentre study of post mortem findings, Equine Veterinary Journal 43(3): 324-331.
- Robinson NE, Williams KJ, Stack A, Jackson WF and Derksen FJ (2015). Exercise-induced pulmonary haemorrhage: a progressive disease affecting performance? Equine Veterinary Journal 47(3): 339-340.
- Hinchcliff KW, Jackson MA, Brown JA, Dredge AF, O’Callaghan PA, McCaffrey JP, Morley PS, Slocombe RE and Clarke AF (2005). Tracheobronchoscopic assessment of exercise-induced pulmonary hemorrhage in horses, American Journal of Veterinary Research 66(4): 596-598.
