7 Mar 2023
Equine respiratory disorders – an update on severe equine asthma
Nicola Menzies-Gow looks at severe equine asthma (SEA), a naturally occurring chronic inflammatory lower airway disease in adult (seven years or older) equids.

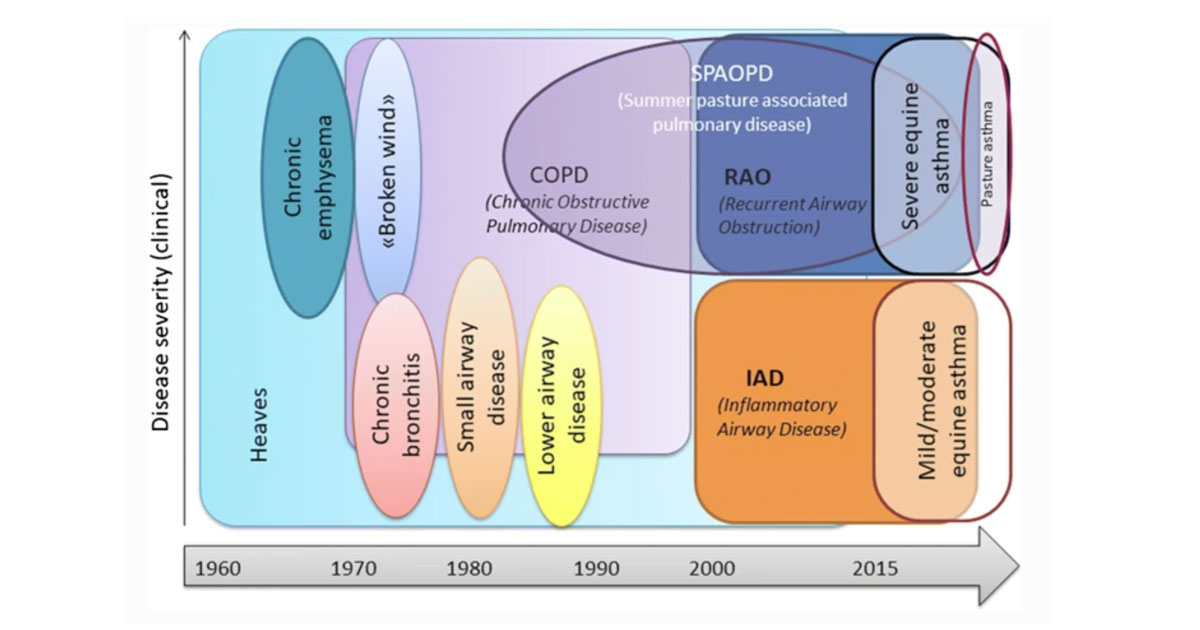
Figure 1. Terminology used to describe asthma-like disease affecting adult horses17.
The term “equine asthma” encompasses all causes of lower airway inflammation. It is divided according to severity into mild-to-moderate equine asthma and severe equine asthma (SEA)1.
The former includes what was previously known as inflammatory airway disease, while the latter includes what was previously known as recurrent airway obstruction (RAO) and summer pasture-associated obstructive airway disease (Figure 1). RAO was previously known as chronic obstructive pulmonary disease and heaves.
SEA is a naturally occurring, chronic inflammatory lower airway disease in adult (seven years or older) equids that has become more prevalent with the domestication of horses. It is most prevalent in the northern hemisphere, where horses are often kept inside and fed hay, with an estimated prevalence of 20%1.
In the 2018 British National Equine Health Survey, respiratory disease was the fourth most common category of disease syndrome recorded, accounting for 7% of reported problems. SEA was the second most commonly reported individual medical problem (4.9% of all syndromes) and accounted for 70% of all respiratory problems. Therefore, SEA is commonly encountered by first opinion equine veterinarians.
In general, horses affected with SEA may also suffer from other allergic diseases, such as insect bite hypersensitivity or atopy.
Pathogenesis
SEA occurs when genetically susceptible individuals are exposed to environments with high concentrations of airborne respirable particles capable of inducing airway inflammation2.
A vast array of antigens has been implicated in the aetiology of SEA, and it is thought that airway inflammation results from the synergistic effect of multiple allergens individuals are susceptible to in unique ways. The disease has been mostly associated with hay feeding and stabling, but can also occur while the horse is out at pasture.
Fungal spores, bacterial endotoxins, forage and storage mites, microbial toxins, peptidoglycans, proteases, pollens and plant debris – as well as inorganic particles – trigger clinical signs of disease. Several fungi – more than 50 species, and especially Aspergillus fumigatus – have been widely recognised as significant risk factors for SEA.
Evidence also exists of a role for novel allergens, including new species of fungi, mites, pollen and arthropods, but also that of latex proteins. These allergens deposit in the bronchioles, triggering a pro-inflammatory and hypersensitivity cascade manifesting in the airways as a consistent accumulation of neutrophils, excess mucus production, bronchospasm and airway remodelling.
While human asthma is divided into two major types according to cytokine profile – T helper-2 (Th2) and non-Th2-type asthma – the precise cytokine profile of SEA remains unclear, with a multitude of reports pointing to either a Th1, Th2, Th17 or mixed mediated response. Therefore, it is likely that multiple types of SEA exist, associated with varying cytokine profiles and immunological pathways that converge into the same clinical phenotype.
Genetic background
Although the heritability of SEA has been shown in several horse breeds3 and a familial aggregation has been ascertained, age and external factors – such as the environment – increase the likelihood of expressing the disease. The chromosome region ECA13 has been associated with SEA in one family of Swiss warmbloods, while region ECA15 has been implicated in a different family of the same breed. The inheritance mode differed between the two families, being autosomal recessive in the first family and autosomal dominant in the second4.
Additionally, in the first family of horses, the interleukin-4 receptor (IL-4R) gene and its neighbouring regions in ECA13 appeared to contribute to disease in some individuals.
In humans, polymorphic differences in the IL-4R α chain gene play an important role in the development of asthma. In a genome-wide association study, the gene responsible for the TXNDC11 protein, also located in the ECA13 region, has been linked to SEA5.
In humans, TXNDC11 controls the expression of MUC5AC mucin, which has been shown to play a significant role on airway hyperreactivity in mice. In SEA-affected horses, MUC5AC is upregulated6 – and, therefore, potentially contributing to the mucus plugging observed in the disease.
The analysis of genomic copy number variants did not reveal any relevant variant regions that could be associated with SEA, although a copy number loss was reported on chromosome 5 involving the gene NME77. The expression of this gene is necessary for ciliary function in the lungs, and so may be involved in SEA.
Also, using RNA sequencing technique, a single point substitution was detected in the PACRG and RTTN genes in asthmatic horses, predictively altering their proteins, which are related to ciliary function8. In a gene set enrichment analysis of the bronchial epithelium after hay dust exposure, asthmatic horses presented upregulated genes of the E2F transcription factor family, which contribute to cell cycle regulation9.
Overall, these studies illustrate the complex genetic heterogeneity of SEA, which most likely results from the interaction of different genes.
Airway remodelling
SEA is characterised by bronchial remodelling, which is only partially improved by a prolonged period of disease remission induced by therapy or antigen avoidance strategies.
While descriptive changes in the anatomy and structure of the lungs of asthmatic horses date back to some decades ago, and have been confirmed over time, it is in more recent times that these alterations have been quantified. This has corroborated the initial observations that the magnitude of airway remodelling varies along the bronchial tree, and it is most marked peripherally (airways less than 2mm in diameter).
Most remodelling data collected in asthmatic horses concerns airway smooth muscle (ASM), which is reasonable, given its importance in disease pathophysiology. The available evidence suggests that ASM mass is increased both in central/intermediate and peripheral airways, but this process is more accentuated peripherally.
While an average 50% increase in ASM mass has been reported in central airways of asthmatic versus control horses, up to a 300% increase has been reported in small peripheral airways. Both neutrophil-mediated hyperplasia and hypertrophy of ASM cells are likely to contribute to this process. This ASM remodelling is not completely reversible by corticosteroid treatment – either alone or in association with a bronchodilator.
The extracellular matrix (ECM) lying within the lamina propria of the airways is also altered in SEA – both in the central and peripheral airways. The total collagen content is increased in the peripheral airways of asthmatic horses and correlates with pulmonary resistance during disease remission. However, the use of corticosteroids and β2-agonists have been shown to nearly normalise the ECM remodelling in asthmatic horses.
It remains to be clarified whether the mechanical properties of the asthmatic airways in which remodelling has been reversed and of the healthy airways are the same.
Clinical signs
The clinical signs of SEA are varied and affected horses can present with combinations of:
- nasal discharge
- coughing
- tachypnoea at rest
- dyspnoea at rest
- exercise intolerance
Diagnosis
Diagnosis of SEA is based on signalment, history, physical examination and clinical signs, and can be confirmed through cytological examination of a sample obtained from the lower respiratory tract. Examples are tracheal wash (TW) or bronchoalveolar lavage (BAL) fluid.
SEA is characterised by increased tracheobronchial mucus and non-degenerate inflammatory cells in the respiratory tract samples. Historically, cut-off values for neutrophil percentage have been set at 20% for TW and 5% for BAL. A new cut-off value of more than 10% neutrophils for BALF cytology has been proposed lately for the diagnosis of airway inflammation to allow more variability caused by the variation in sampling techniques10.
Although lung function testing can accurately detect SEA, such equipment is unavailable to most field practitioners.
TW wash versus BAL
Generally, a TW is considered to give a better representation of the whole lung than a BAL and, therefore, is often preferred in cases where an infectious disease is suspected, because secretions from the affected lung areas will collect in the trachea.
The advantages of TW in clinical practice are its lower costs and the potential avoidance of drug withdrawal times that arise from sedation and local anaesthesia, often administered during the BAL procedure to restrain the horse and suppress coughing. On the other hand, a BAL is recommended in suspected diffuse non-bacterial lung disease, and it is considered to be a more sensitive technique for detecting lower airway inflammation11.
Furthermore, BALF cytology may correlate better with lung histopathology results. TW and BAL neutrophil percentages correlate in some, but not all, studies. Therefore, in cases of uncertain aetiology, performing both diagnostic tests simultaneously has been suggested to achieve accurate diagnosis.
Management
The management of SEA is multifaceted and includes non-pharmacologic and pharmacologic strategies. As no cure for SEA exists, the mainstay of treatment is through minimising the exposure to environmental allergens, and managing the symptoms of bronchospasm and airway inflammation with medical therapy.
Non-pharmacologic management
Antigen avoidance is the strategy of choice to manage SEA and is achieved by modifying the diet – mainly by replacing dry hay with less dusty hay alternatives and reducing the environmental antigenic exposure.
If the horse is allergic to pollen in the field, it should be kept inside with dust-free management. If the allergens are dust and moulds found inside, the horse should be on full-time pasture turn out. Horses in a controlled dust-free environment have been shown to achieve clinical remission without medical treatment12.
However, it is recognised that creating a dust-free environment can be challenging and not feasible for all owners. In addition, horses with concurrent systemic diseases, such as laminitis or arthritis, may not be able to cope with the required husbandry changes.
As such, pharmacological management plays a major role in the symptomatic management and remission of patients with SEA.
Suppression of pulmonary inflammation
NSAIDs have limited-to-no value in SEA, and so corticosteroids are the mainstay of treatment to reduce pulmonary inflammation. They can be administered via nasal inhalation or systemically13. Horses are ideal candidates for inhaled medication as they are obligate nasal breathers with a large tidal volume.
Inhaled corticosteroids include beclomethasone dipropionate, fluticasone propionate, budesonide and a newer drug, ciclesonide14, which is licensed for the alleviation of clinical signs of SEA. Dexamethasone and prednisolone are systemic corticosteroid therapies shown to improve clinical signs. They can be delivered as an IV injection or orally consumed as a powder or tablet, and preparations are licensed for the treatment of inflammatory and clinical parameters associated with SEA, or for the treatment of inflammatory conditions in horses.
Meta-analysis evaluating the effects of corticosteroids on clinical score and lung function in SEA did not detect a difference in the magnitude of improvement between systemic and inhaled preparations15. However, when inhaled corticosteroid preparations are directly compared to appropriate systemic administration, the latter is never inferior, but often superior16.
Furthermore, it is expected that systemic administration should result in a faster and greater improvement in horses suffering from severe airway obstruction in which mucus accumulation, bronchospasm and cough might impair lower airway deposition of inhaled medication13.
Another potential benefit of corticosteroids in the clinical management of airway obstruction is the prevention of tachyphylaxis to β2-adrenergic agonists. Dexamethasone prevented the clenbuterol-induced β2-adrenergic receptor density downregulation on equine lymphocytes. Therefore, it is commonly recommended to use β2-adrenergic agonists and corticosteroids concomitantly to prevent reduced efficacy of the bronchodilator.
The side effect profile of corticosteroids is important to consider. For example, obese horses may not be suitable candidates for systemic corticosteroid administration due to an increased laminitis risk. However, it should be remembered that ciclesonide is a prodrug that is de-esterified in the lung to the active metabolite desisobutyryl-ciclesonide (des-CIC).
With a 100 to 120-fold higher glucocorticoid receptor binding affinity than ciclesonide, and 12 times higher than dexamethasone, des-CIC is the effective drug that elicits typical glucocorticoid effects at the site of activation in the airways, and, therefore, significantly reduces the potential for systemic adverse effects.
Additionally, its high protein binding in circulation likely limits its systemic effects, as corticosteroids linked to proteins do not activate their receptors. Therefore, it might be the drug of choice in animals at risk of corticosteroid-associated laminitis.
While the improvement in lung function is evident within a few days of commencement of treatment, inhaled and systemic corticosteroids have limited residual effect after cessation of the treatment. For this reason, when corticosteroid use is required it should be combined with modifications of dietary and environmental conditions to reduced allergen exposure.
Bronchodilation
Bronchodilators are used in conjunction with environmental change and corticosteroids. They provide more immediate relief of the bronchospasm and, therefore, the clinical signs. Available options include β2-agonists and anticholinergic agents.
Clenbuterol is a β2-agonist available as a solution for IV injection or as granules/syrup for oral administration. It is licensed for the treatment of respiratory disease in horses where airway obstruction due to bronchospasm and/or accumulation of mucus is a contributing factor, and improved mucociliary clearance is desirable.
Potential side effects associated with a lack of β2-receptor selectivity include sweating, muscle tremors and excitement. In addition, long-term therapy can be associated with tachyphylaxis due to receptor downregulation and a need to increase the dose, resulting in an increased risk of side effects. Alternative non-licensed preparations for inhaled use include albuterol and salmeterol.
Anticholinergic drugs include atropine, hyoscine and ipratropium bromide. Atropine can be administered intravenously to relieve acute dyspnoea as a one-off, but the potential for adverse effects on gastrointestinal motility should be considered. A safer alternative IV option is hyoscine.
Finally, ipratropium bromide is available as an inhaled preparation. None of the available preparations are licensed for use in the horse, and so are used under the prescribing cascade.
Reduction in mucus accumulation
β2-agonists have the additional beneficial effect of increasing mucociliary clearance and, therefore, reducing accumulation.
Dembrexine is available as an oral powder that is licensed for the symptomatic treatment of acute, sub-acute and chronic respiratory disease of the upper and lower respiratory tract, where an abnormal amount of mucus of increased viscosity is present.
References
- Couëtil L et al (2020). Equine asthma: current understanding and future directions, Front Vet Sci 7: 450.
- Simoes J et al (2022). The immune mechanisms of severe equine asthma – current understanding and what is missing, Animals (Basel) 12(6): 744.
- Gerber V et al (2009). Mixed inheritance of equine recurrent airway obstruction, J Vet Intern Med 23(3): 626-630.
- Swinburne JE et al (2009). A whole-genome scan for recurrent airway obstruction in warmblood sport horses indicates two positional candidate regions, Mamm Genome 20(8): 504-515.
- Schnider D et al (2017). A genome-wide association study for equine recurrent airway obstruction in European warmblood horses reveals a suggestive new quantitative trait locus on chromosome 13, Anim Genet 48(6): 691-693.
- Gerber V et al (2003). Mucin genes in horse airways: MUC5AC, but not MUC2, may play a role in recurrent airway obstruction, Equine Vet J 35(3): 252-257.
- Ghosh S et al (2016). Analysis of genomic copy number variation in equine recurrent airway obstruction (heaves), Anim Genet 47(3): 334-344.
- Tessier L et al (2018). Sequence variant analysis of RNA sequences in severe equine asthma, PeerJ 6: e5759.
- Tessier L et al (2018). Gene set enrichment analysis of the bronchial epithelium implicates contribution of cell cycle and tissue repair processes in equine asthma, Sci Rep 8(1): 16,408.
- Couëtil LL et al (2016). Inflammatory airway disease of horses – revised consensus statement, J Vet Intern Med 30(2): 503-515.
- Hoffman AM (2008). Bronchoalveolar lavage: sampling technique and guidelines for cytologic preparation and interpretation, Vet Clin North Am Equine Pract 24(2): 423-435, vii-viii.
- Simoes J et al (2020). Owner compliance to an environmental management protocol for severe equine asthma syndrome, J Equine Vet Sci 87: 102,937.
- Mainguy-Seers S and Lavoie J-P (2021). Glucocorticoid treatment in horses with asthma: a narrative review, J Vet Intern Med 35(4): 2,045-2,057.
- Pirie RS et al (2021). Inhaled ciclesonide is efficacious and well tolerated in the treatment of severe equine asthma in a large prospective European clinical trial, Equine Vet J 53(6): 1,094-1,104.
- Calzetta L et al (2017). Pharmacological treatments in asthma-affected horses: a pair-wise and network meta-analysis, Equine Vet J 49(6): 710-717.
- Robinson NE et al (2009). Fluticasone propionate aerosol is more effective for prevention than treatment of recurrent airway obstruction, J Vet Intern Med 23(6): 1,247-1,253.
- Bullone M and Lavoie J-P (2020). The equine asthma model of airway remodeling: from a veterinary to a human perspective, Cell Tissue Res 380(2): 223-236.
