18 Jun 2018
Investigating lower airway disease in horses: part 2
Lara Gosling, Jonathon Dixon and David Rendle discuss using thoracic radiography and ultrasonography to identify and monitor progression of this disease.
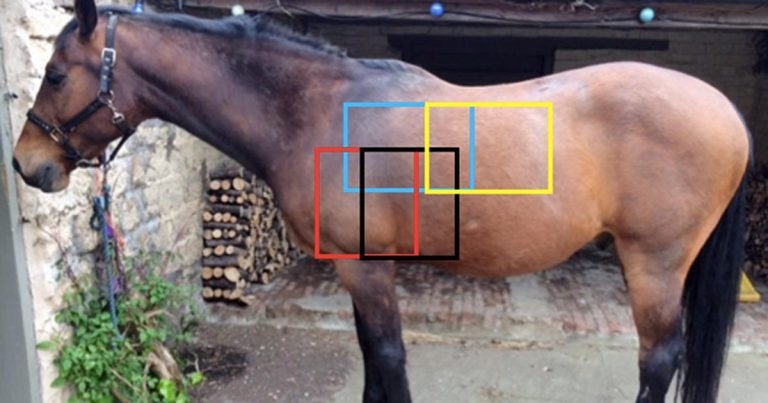
Figure 1. The four overlapping regions that should be radiographed to image the lung fields; cranioventral region shown in red, caudoventral in black, craniodorsal in blue and caudodorsal in yellow.
Lower respiratory tract disease is common in equine practice and sampling is central to diagnosis in most cases, as discussed in part one of this article.
This part discusses conventional imaging modalities that can be used in further investigating lower respiratory tract disease. Thoracic radiography and ultrasonography are often underused techniques that can be performed in clinical practice and both provide valuable information.
While sampling the airways offers information on bacterial involvement and inflammatory changes at a cellular level, ultrasonography and radiography provide an overview of gross anatomy and pathology of the lung and other intrathoracic structures, as well as allowing localisation of more specific lesions (Figure 1). Imaging the thorax provides both diagnostic and prognostic information, as well as being a useful tool for monitoring disease progression.
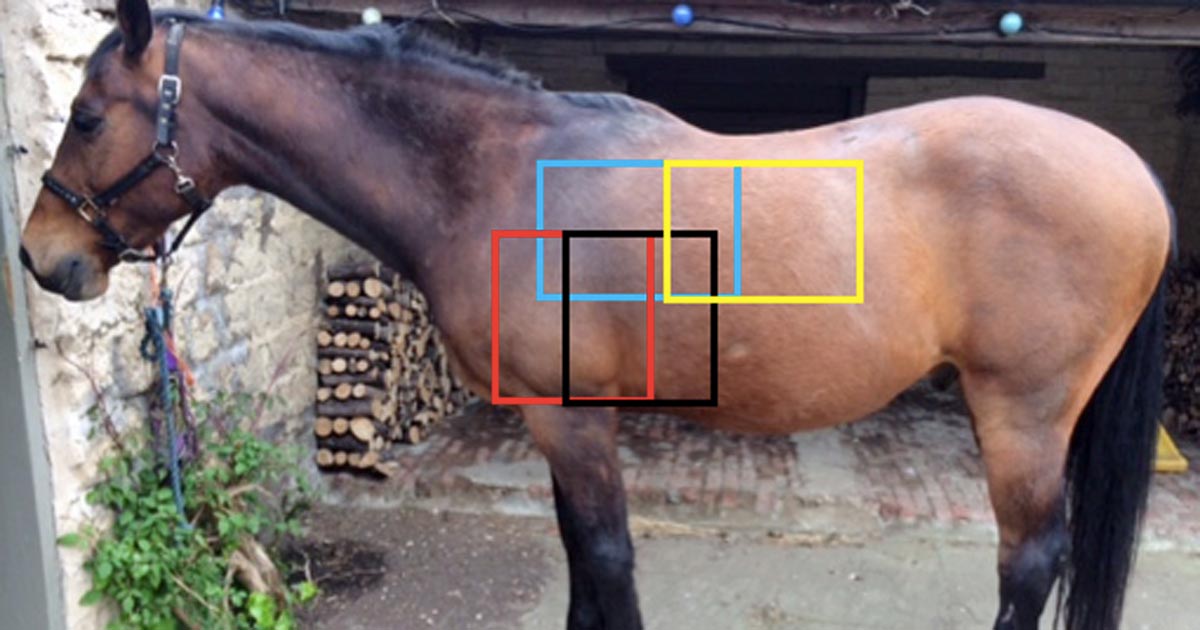
and caudodorsal in yellow.
Thoracic radiography
Thoracic radiography is an underused technique in equine practice – with modern equipment and correct technique it can provide valuable information on the health or disease of the lower respiratory tract.
Radiography is useful in identifying horses with pulmonary parenchymal disorders or mediastinal disease and can identify gas or fluid within the pleural space. Pathology may be serially monitored radiographically once diagnosed to aid in assessment of response to treatment. Radiography may also provide a means of evaluating the lower airways when airway sampling is contraindicated due to respiratory distress.
The major benefit of thoracic radiography over ultrasonography is examination is not limited to the superficial aspects of the pulmonary parenchyma. The findings of radiography can be normal in some horses with respiratory disease, so the absence of radiographic changes does not preclude the presence of significant pathology.
Technique
The thorax is divided into four overlapping radiographic regions for the purposes of radiography: craniodorsal, caudodorsal, cranioventral and caudoventral. Laterolateral radiographs should be taken from both sides to overcome the effects of magnification and blurring that results from both normal respiratory motion – and the large distance between the pulmonary parenchyma on the side of the horse nearest the generator and the plate on the far side of the horse.
Sedation facilitates positioning of the horse and reduces risk of movement. The horse should stand square with the forelimbs extended forwards to expose the cranioventral thorax. A large plate (ideally 35cm×43cm) should be placed adjacent to the horse’s thorax, preferably with a grid or digital grid to minimise scatter.
A short exposure time (less than 0.2 seconds) will minimise movement blur associated with breathing, though sometimes this is only possible with a gantry-mounted high-output generator. If possible, images should be obtained at maximal inspiration. High radiation exposures are often needed, so the detector should not be handheld – rather a plate stand should be used, which will also aid in reduction of movement blur. For a cranioventral radiograph, pulling the limb ipsilateral to the x-ray generator forwards will result in a sharper image, as degradation due to scatter from triceps muscle will be reduced.
Normal radiographic anatomy
Normal radiographic features are shown in Figure 2.
Abnormal radiographic findings
Abnormalities can be categorised loosely into several types of visible parenchymal change; bronchial change, interstitial change and alveolar change. These changes frequently occur in combination.
Alveolar pattern
An alveolar pattern is seen most commonly in the ventral and caudoventral pulmonary parenchyma, and occurs when alveoli either become filled with fluid or are collapsed in association with pulmonary consolidation or atelectasis. An alveolar pattern is characterised by irregular regions of increased soft tissue opacity (Figure 3).
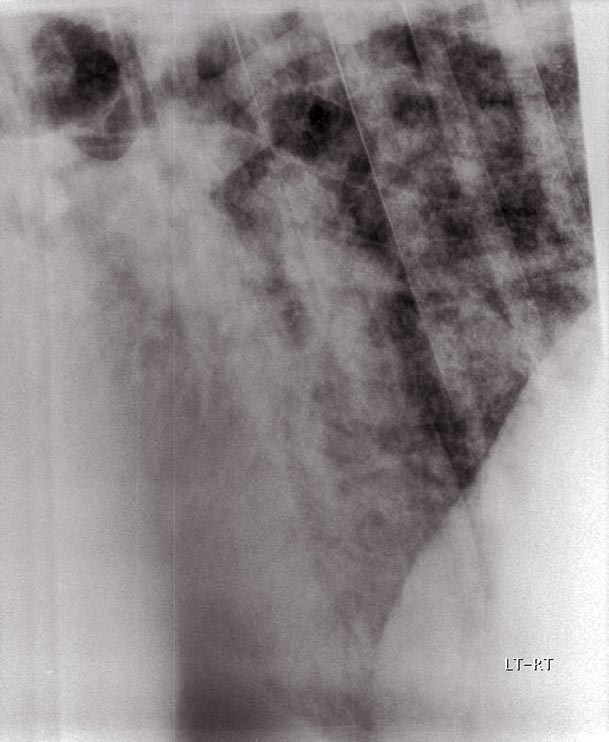
Air bronchograms may be evident as round or linear branching radiolucencies, and are a characteristic feature of an alveolar pattern. The margins of lung lobes may be evident if specific lobes are affected and have a different radiodensity from neighbouring lobes (“lobar sign”); however, this is uncommon in horses. Recumbency may cause atelectasis, but this is unlikely to be a factor in adult horses with respiratory disease.
Ventral lung consolidation caused by pneumonia typically appears as a soft tissue opacity with an alveolar pattern on radiography. It is common for the opacities to be partially superimposed over the margins of the cardiac silhouette, so care must be taken to critically evaluate this region. Bronchopneumonia, pulmonary oedema (either cardiogenic or non-cardiogenic), haemorrhage and neoplastic infiltration can also lead to an alveolar pattern.
Interstitial pattern
Assessing the radiodensity of the interstitium is subjective and complicated by the variation present in normal horses (Barakzai and McAllister, 2007).
Pulmonary disease may cause a uniform or non-uniform (unstructured) increase in the background radiodensity of the lung tissue, which can result in reduced definition of the margins of the pulmonary vessels and bronchial walls. These changes are the result of fluid or cellular material occupying the interstitial space. A structured interstitial pattern describes radiodense nodules (less than 2cm) or masses (more than 2cm) that should not be confused with ”end-on” pulmonary vessels. These nodules or masses are distinct, localised areas in which fluid or cellular material is present.
Interstitial pneumonia may be associated with a structured or unstructured pattern (Figure 4a). In this condition, radiography is the most useful non-invasive method of diagnosis and monitoring (Mair, 2007). An interstitial or bronchial pattern may be seen with chronic marked equine asthma (formerly recurrent airway obstruction), and radiography in these cases helps rule out other possible causes of respiratory distress. A nodular interstitial pattern may be caused by pulmonary abscessation, granulomatous disease or equine multinodular pulmonary fibrosis (Figure 4b).
Bronchial pattern
Increased opacity or thickness of the bronchial walls is termed a bronchial pattern. In cross-section, the normal bronchi appear as radiodense rings with a radiolucent centre (often referred to as ”doughnuts”). In the longitudinal section paired ,radiodense lines run in parallel (often referred to as ”tram lines”) and are evident tapering further into the periphery than would be evident in normal horses (Barakzai and McAllister, 2007). The failure of tram lines to taper towards the periphery may indicate bronchial dilation, such as bronchiectasis.
A bronchial pattern can be a normal finding in older horses and occurs as a result of mineralisation of the airways. A pathologic bronchial pattern occurs if significant lower airway inflammation is present, and fluid and inflammatory cells accumulate in the loose interstitium surrounding the airways (Wilkins, 2003).
A bronchial pattern is particularly common in horses with equine asthma, especially in chronic cases where peribronchial fibrosis has occurred; however, it may also be present in cases of interstitial pneumonia with marked peribronchial oedema. The extension of oedema and cellular infiltrates into the tissues surrounding the bronchi gives rise to a bronchointerstitial pattern.
Pneumothorax
Retraction of the dorsal edge of the pulmonary parenchyma ventrally away from the margins of the vertebral bodies and diaphragm are the characteristic signs of a pneumothorax. This is typically evident as a linear or curved radiodense area in the dorsal thorax (Figure 5).
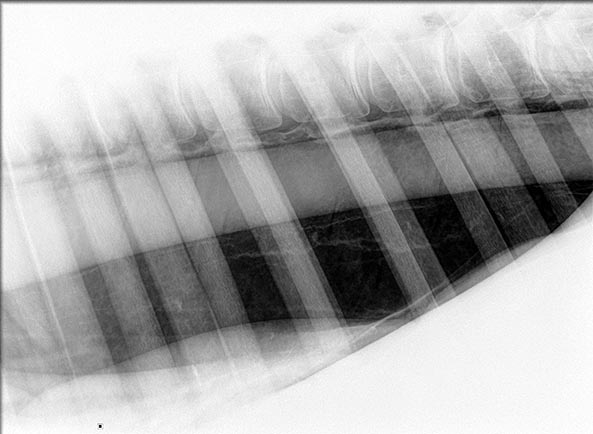
If pneumothorax is bilateral, the normal lung tissue appearance above this line will be lost, with no visible pulmonary vessels or bronchi. Radiographs may help in identifying the underlying cause of the pneumothorax, such as a penetrating foreign body (Byars and McGorum, 2007). If pleural fluid is also present, a distinct horizontal gas-fluid interface will exist between the radiolucent gas-filled space and the radiopaque fluid.
Pneumomediastinum/pneumopericardium
The presence of gas around the great vessels or trachea indicates pneumomediastinum, which can result from penetrating thoracic injuries, bacterial infection, or the diffusion of gas from other regions. Tears in the oesophagus or trachea are uncommon, but are potential causes of pneumomediastinum. The presence of gas within the pericardial sac is very uncommon.
Pleural effusion
In adult horses, it is estimated a minimum of 500ml of fluid needs to be present within the pleural space before it can be seen radiographically as a soft tissue opacity in the ventral thorax obscuring the cardiac silhouette and merging with the diaphragm (Barakzai and McAllister, 2007).
Larger volumes of fluid may displace the lungs dorsally. If pleural effusion is seen on radiography, it should be drained prior to radiography being repeated, as the presence of pleural fluid obscures the soft tissues of the ventral thorax (Lugo and Carr, 2012). Horses with pleural effusion of no defined cause should be evaluated carefully, both radiographically and ultrasonographically, for the presence of a mediastinal mass.
Mediastinal masses
Mediastinal masses are most commonly the result of abscessation or neoplasia, particularly lymphoma. Differentiating abscessation from neoplasia is obviously important in determining prognosis, but can be challenging. Mediastinal masses can sometimes be seen radiographically cranial to the cardiac silhouette as soft tissue opacities that displace the normal anatomical structures (Dunkel et al, 2013).
The position of the trachea should be assessed carefully, as its dorsal displacement is suggestive of a space-occupying lesion within the mediastinum, though this finding can be subtle. Mediastinal masses may be associated with pleural effusion, which will obscure the ventral thorax on radiography. As described previously, the effusion should be drained under ultrasound guidance prior to radiography being repeated.
Thoracic ultrasonography
Thoracic ultrasonography can be performed quickly and easily in the field with any modern ultrasonography equipment. Ultrasonography is more sensitive than radiography for identifying the presence of pleural effusion (Lugo and Carr, 2012). The sensitivity of ultrasonography for detection of small volume-induced pneumothorax has been calculated at 84%, this being superior to radiography (48%; Partlow et al, 2017).
Ultrasonography is also the best means of evaluating the peripheral pulmonary parenchyma and pleural surfaces, and enables localisation and lateralisation of lesions more readily than radiography.
Technique
As with any ultrasonographic examination, good skin contact is key to obtaining high-quality images. The hair should be clipped if the horse has a thick coat, or at least cleaned prior to liberal application of alcohol and/or coupling gel. A 5MHz curvilinear or linear probe provides a good balance of resolution and penetration.
A high frequency linear transducer will provide the best anatomic detail; however, in cases with significant pleural disease or effusion, the maximum depth possible may not allow detailed examination of all abnormal structures. Both hemithoraces should be scanned systematically, imaging each rib and intercostal space from dorsal to ventral, and moving from cranial to caudal across the entire lung field (Rosenstein, 2007).
Normal anatomy
The presence of air in normally inflated lungs gives a highly echogenic boundary line preventing examination of deeper structures (Figure 6). The interface of the pleural surfaces is evident as a bright hyperechoic line that slides back and forth during inhalation and exhalation; the “gliding lung sign” (Partlow et al, 2017).
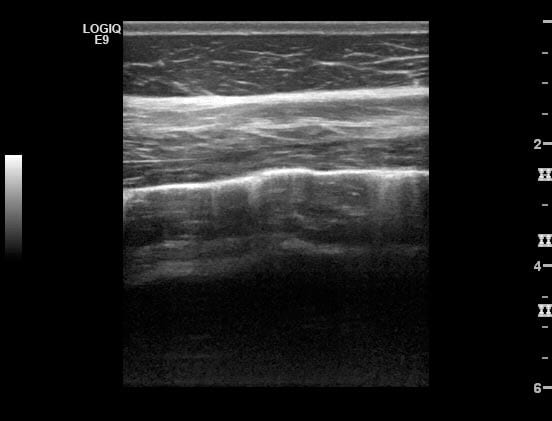
A very small volume of anechoic pleural fluid separating the lung surface from the parietal pleura can be considered a normal finding (Ainsworth and Cheetham, 2010) and at its ventral tip the lung may be seen to deviate slightly away from the body wall in normal horses, with the space between the pleural surface being occupied with anechoic fluid. Ribs are evident as bright curvilinear echoes causing an acoustic shadow artefact.
Abnormal findings
Pleuritis
Irregularity of the pleural surface causes reverberation artefacts often referred to as ”comet tails”. Comet tails are occasionally seen in horses that do not have pulmonary disease, especially at the ventral margin of the lung; however, the presence of multiple, large or widespread comet tails is indicative of pleural disease that is invariably secondary to disease of the underlying parenchyma.
Pleural effusion
Pleural effusion typically results from bacterial pleuropneumonia or thoracic neoplasia. Ultrasonography is the most sensitive imaging modality for diagnosis and characterisation of pleural effusion, and allows imaging-guided thoracocentesis (Lugo and Carr, 2012). A triangle of fluid between the ventral lung and thoracic wall or diaphragm is characteristic of a pleural effusion, and the fluid may displace the lung tissue axially and dorsally.
The echogenicity of the fluid varies with its nature (Figures 7 and 8); the echogenicity increases as the amount of protein and inflammatory cells increase. The presence of hyperechoic flecks and associated drop out suggests the presence of gas that might be caused by anaerobic bacteria (Ainsworth and Cheetham, 2010) or pneumothorax. With extensive pulmonary disease a possibility exists of pneumothorax developing from bronchopulmonary fistulae.
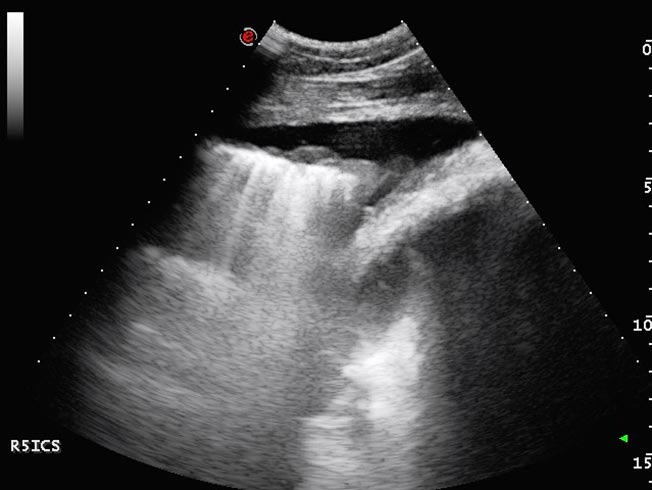
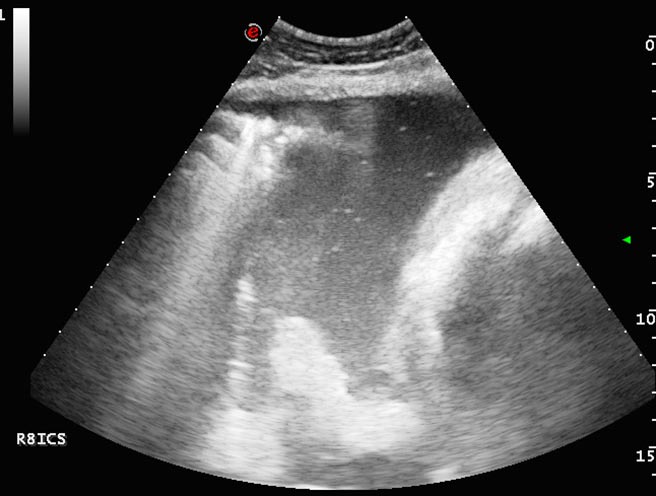
Small echogenic particles or motile hyperechoic linear structures within the pleural fluid indicate the presence of fibrin that, with time, often form adhesions between pleural surfaces. As fibrin deposition becomes more extensive, thick bands of fibrin separated by pockets of fluid may develop and take on a honeycomb appearance (Rosenstein, 2007; Nykamp, 2013; Figure 9). Extensive fibrinous material or loculation complicates treatment as it frustrates attempts to drain or lavage the pleural space and, consequently, is associated with a poorer prognosis (Tomlinson et al, 2015).
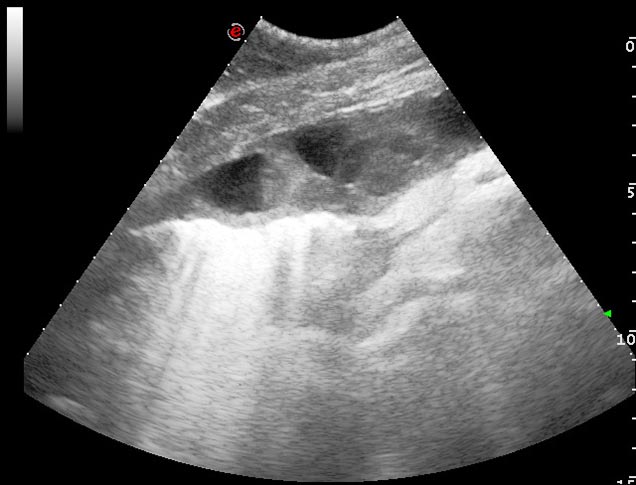
Progression of pleuropneumonia with increased volumes of pleural effusion can also lead to pulmonary consolidation (Lugo and Carr, 2012). Pulmonary consolidation can be seen ultrasonographically as anechoic or hypoechoic fluid bordering dense lung tissue of moderate echogenicity with visible hypoechoic blood vessels, which has a similar appearance to liver tissue (Figure 10).
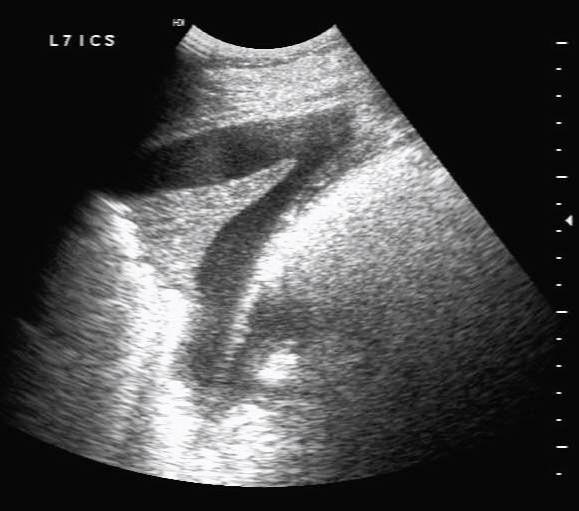
Where pleural effusion is identified, a thorough ultrasonographic examination of the thoracic cavity is indicated to identify the presence of associated abscesses or neoplasia. The likely cause is often evident from the history. If neoplasia is identified, ultrasonographic examination of the abdominal cavity may reveal peritoneal effusion and intra-abdominal masses if there is multicentric neoplasia.
Pneumothorax
On ultrasonographic examination, gas in the pleural space appears as a non-moving gas artefact that obscures visualisation of normal respiratory movement at the interface of the two pleural surfaces.
Ultrasonography was determined to be more sensitive than radiography in identifying small-volume pneumothorax (Partlow et al, 2017); however, pneumothorax is easier to identify on radiography if a large amount of air is present. If pneumothorax is identified or suspected then thoracic radiography is the best means of determining its severity.
Atelectasis and consolidation
Atelectasis describes collapse of the alveoli and loss of normal aeration, and when it occurs, the normal reverberation artefact at the pleural surface is lost (Figures 11 and 12). The hallmark of atelectasis is loss of volume of the pulmonary structures, a feature difficult to quantify in equids. By contrast, consolidation occurs where the gas within the alveoli is replaced with fluid or cellular material without any loss of volume.
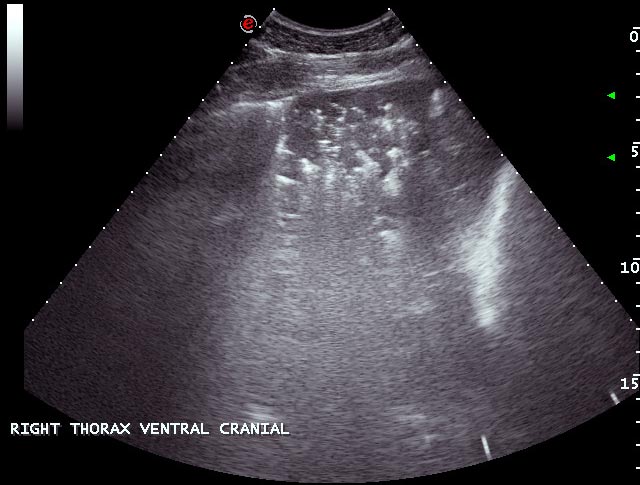
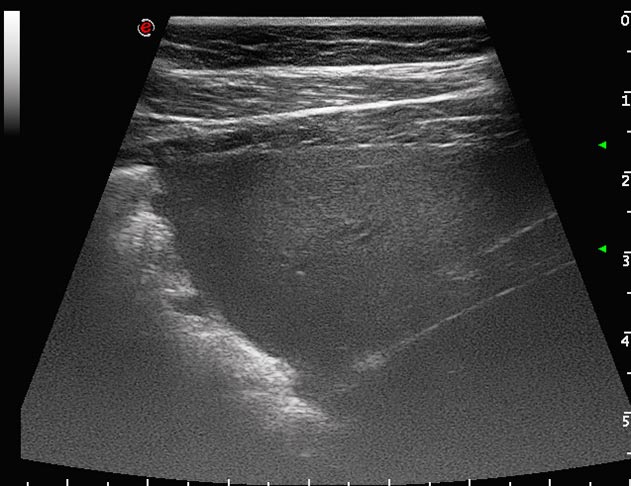
Consolidated, non-aerated lung has a variable echogenicity with visible hypoechoic blood vessels; however, unlike normally aerated lung, ultrasound waves penetrate the parenchyma, revealing internal architecture. Hyperechoic foci may be evident if air remains within some of the larger bronchi or bronchioles. The appearance of consolidated lung is not dissimilar to that of liver, for which it can be mistaken if care is not taken and the position of the diaphragm is not identified.
Both atelectasis and consolidation may occur following prolonged recumbency, with pneumonia or, most commonly, when fluid in the pleural space prevents expansion of the ventral lung (Figure 10).
Mediastinal masses
Caudal mediastinal masses are visualised most easily when concurrent pleural effusion is present, as this provides an acoustic window through which the mediastinum can be imaged. In a normally aerated lung, lack of parenchymal penetration from the pleural-lung interface precludes visualisation of this area. Cranial mediastinal masses, however, can be visualised at the third intercostal space on the right even with normally aerated lung, as the mediastinum in this area extends cranially to aerated lung tissue (Ainsworth and Cheetham, 2010).
Mediastinal masses are relatively uncommon in the horse; however, differentiating abscesses from neoplasia has implications for treatment and prognosis, so it is important they are identified. The concurrent use of radiography and ultrasonography is recommended if mediastinal disease is suspected (Byars and McGorum, 2007).
Abscesses
Pulmonary abscesses are most commonly seen in foals with Rhodococcus equi infection, but can also be seen in adult horses with pneumonia (Figure 13). Abscesses commonly appear as hypoechoic foci that may have a hyperechoic capsule. In some cases, flocculent material is present within the central portion, and hyperechoic flecks with associated drop-out may indicate gas.
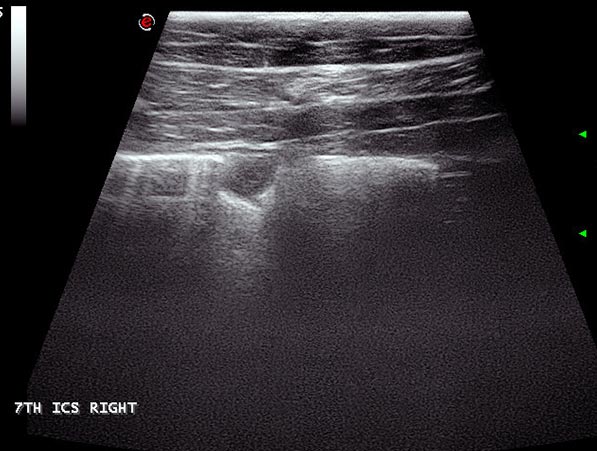
Abscesses are only evident ultrasonographically if they are contiguous with the pleura and no overlying aerated lung is present. It is, therefore, important radiography and ultrasonography are performed concurrently to image all depths if the presence of an abscess is suspected.
References
- Ainsworth DM and Cheetham J (2010). Disorders of the respiratory system. In Reed SM, Bayly WM and Sellon DC (eds), Equine Internal Medicine (3rd edn), WB Saunders, St Louis, US: 296-314.
- Barakzai S and McAllister H (2007). Radiography and radiology of the respiratory tract. In McGorum BC, Dixon PM, Robinson NE and Schumacher J (eds), Equine Respiratory Medicine and Surgery, WB Saunders, Edinburgh: 151-174.
- Byars TD and McGorum BC (2007). Disorders of the thoracic wall, pleura, mediastinum, and diaphragm. In Equine Respiratory Medicine and Surgery, WB Saunders, Edinburgh: 659-676.
- Dunkel B, Gibbs C and Weller R (2013). A fresh approach to equine thoracic radiography, In Practice 35(10): 589.
- Lugo J and Carr EA (2012). Thoracic disorders. In Auer JA and Stick JA (eds), Equine Surgery (4th edn), WB Saunders, St Louis, US: 650-664.
- Mair T (2007). Miscellaneous pulmonary disorders. In McGorum BC, Dixon PM, Robinson NE and Schumacher J (eds), Equine Respiratory Medicine and Surgery, WB Saunders, Edinburgh: 601-615.
- Nykamp SG (2013). The equine thorax. In Thrall DE (ed), Textbook of Veterinary Diagnostic Radiology: Elsevier, St Louis, US: 632-648.
- Partlow J, David F, Hunt LM, Relave F, Blond L, Pinilla M and Lavoie JP (2017). Comparison of thoracic ultrasonography and radiography for the detection of induced small volume pneumothorax in the horse, Veterinary Radiology and Ultrasound 58(3): 354-360.
- Rosenstein DS (2007). Ultrasonography of the respiratory tract. In McGorum BC, Dixon PM, Robinson NE and Schumacher J (eds), Equine Respiratory Medicine and Surgery, WB Saunders, Edinburgh: 175-184.
- Tomlinson JE, Reef VB, Boston RC and Johnson AL (2015). The association of fibrinous pleural effusion with survival and complications in horses with pleuropneumonia (2002–2012): 74 cases, Journal of Veterinary Internal Medicine 29(5): 1,410-1,417.
- Wilkins PA (2003). Lower airway diseases of the adult horse, Veterinary Clinics of North America: Equine Practice 19(1): 101-121.
