24 Jul 2017
Investigating lower airway disease: part 1
Lara Gosling, Jonathon Dixon and David Rendle – in the first of a two-part article – review sampling of the lower airway tract.
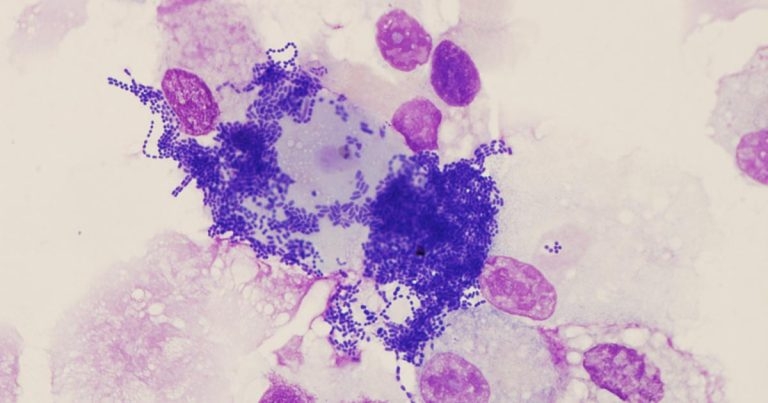
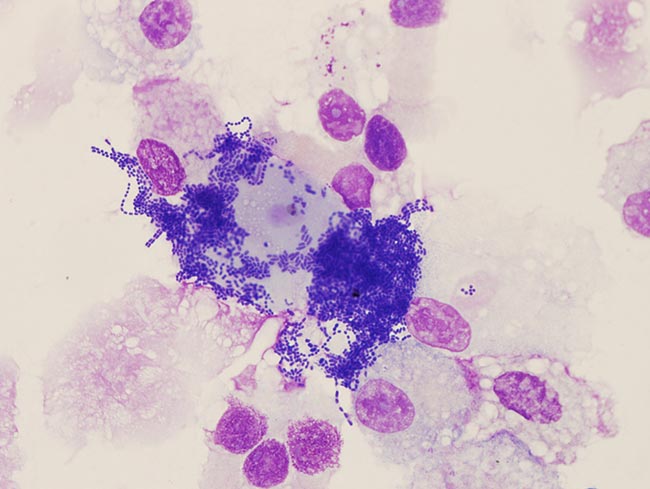
Figure 6. Extensive contamination in a sample of tracheal aspirate fluid.
Lower respiratory tract disease is common in equine practice, with 45% of owners reporting in one study their horse had previously suffered from a cough (Hotchkiss et al, 2007). In a population of leisure horses, 83% had cytological evidence of lower airway inflammation in bronchoalveolar lavage fluid (BALF; Wasko et al, 2011).
In the UK, the National Equine Health Survey reported a respiratory disease prevalence of 5% (Slater, 2014). The spectrum of inflammatory lower airway disease, now reclassified as equine asthma (Couëtil et al, 2016), ranges from mild equine asthma (formerly inflammatory airway disease) – in which clinical signs are not apparent at rest – to marked equine asthma (formerly recurrent airway obstruction; heaves) where there may be marked respiratory distress (Figure 1).

Lower airway washes
Indications for sampling
Lower airway washes should be considered for all cases of lower respiratory disease and may also be indicated in cases presenting with pyrexia of unknown origin or poor performance. A tracheal wash or aspirate samples the larger airways, while a bronchoalveolar lavage samples the smaller, more distal airways. Performing both techniques in one sampling period is strongly recommended, as each provides information the other cannot. Identification of neutrophilia in BALF is the gold standard test for diagnosis of lower airway inflammation (Couëtil et al, 2016; Cian et al, 2015).
Poor correlation exists between BALF cytology and both tracheal aspirate fluid (TAF) cytology and tracheal mucus grading (Gerber et al, 2003; Fraipont et al, 2011), so visualisation and sampling of the trachea alone is not sufficient to fully assess lower airway inflammation.
Respiratory tract sampling may not be appropriate in cases with acute respiratory distress and, in such cases, should be postponed until respiratory function improves.
Endoscopic airways assessment
If respiratory disease has not been localised to the lower respiratory tract, thorough assessment of the nasal passages, nasopharynx, guttural pouches, trachea and carina should be performed. Laryngeal function (unsedated), discharge from either the sinus drainage angle or guttural pouches, and pharyngeal lymphoid hyperplasia should all be noted. After a tracheal aspirate has been collected, the endoscope can be advanced to the distal trachea so the carina and mainstem bronchi can be assessed.
Grading mucus in the trachea
The quantity of mucus in the trachea is assessed endoscopically (Figure 2) and a subjective grade (Gerber et al, 2004) applied as follows:
- 0 No mucus
- 1 Multiple small blobs of mucus
- 2 Large blobs of mucus
- 3 Streams of mucus
- 4 Pooling mucus
- 5 Extreme/profuse mucus pooling ventrally with blobs dorsolaterally
Due to the efficiency of the mucociliary escalator in normal horses, little or no mucus should be present in a healthy respiratory tract.
Tracheal aspirate
Tracheal aspirates may be collected via transendoscopic and transtracheal methods. A transtracheal aspirate is indicated where a sterile sample needs to be obtained – for example, cases of pleuropneumonia. However, it is invasive and carries a risk of infection at the incision site, so is less commonly performed – especially when good transendoscopic technique minimises the risk of sample contamination (Christley et al, 1999).
A transendoscopic tracheal aspirate allows visualisation of the airways and grading of the mucus present (Allen and Franklin, 2007). It is essential the tip of the endoscope remains proximal to the fluid pool prior to sampling to prevent contamination with oropharyngeal bacteria.
To perform a tracheal aspirate:
- Appropriately restrain the horse using sedation, a twitch or stocks, as appropriate.
- Position the endoscope in theproximal trachea.
- Instil 20ml to 30ml of warm, sterile saline into the trachea via a catheter.
- Withdraw the catheter and slowly advance the endoscope until the pool of saline is visualised in the tracheal sump.
- Advance the catheter into the pool of saline and aspirate the sample, keeping the scope proximal to the fluid.
- Place the aspirated fluid into a container containing cytospin (or an EDTA blood tube) for cytological examination and into a plain tube for bacterial culture.
Once the sample has been collected, the catheter can be withdrawn, and the carina and mainstem bronchi can be examined. If a BAL is also to be performed, lidocaine can be instilled around the tracheal bifurcation and larynx before the scope is withdrawn.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) provides more reliable information on peripheral airway disease and correlates well to clinical findings in inflammatory airway disease (Hoffman, 2008). Variability in TAF cytology results can be significant, with little correlation between TAF cytology and pulmonary histopathology in cases with lower airway disease (Larson and Busch, 1985; Winder et al, 1989), so BAL should always be performed in cases with suspected lower airway inflammation (unless the animal is in acute respiratory distress). Bacteriological assessment is rarely performed on BAL samples due to inevitable contamination as the tube passes through the oropharynx (Hodgson and Hodgson, 2007).
Performing a BAL requires the horse to be sedated, ideally with butorphanol included in the sedation protocol for its antitussive effects (Cavanagh et al, 1976). Administration of lidocaine either via an endoscopic catheter or through the nasobronchial tube into the lower airways may reduce coughing. A BAL can be performed using a cuffed nasobronchial tube or via an endoscope. Coughing should be expected as the endoscope/tube reaches the carina and the owner should be warned of this prior to commencing the procedure. Endoscopic BAL may be preferred if a specific region is to be sampled. However, in equine asthma, pathology is typically distributed equally throughout the pulmonary parenchyma and, although regional aspiration may yield samples with slightly differing cytological profiles, this is unlikely to alter the clinical interpretation (Mazan, 2015; Kutasi et al, 2011).
After a BAL, the horse should be rested for 24 hours.
Performing BAL using a cuffed nasobronchial tube
- Pass the nasobronchial tube via the ventral meatus into the trachea. With the head held outstretched, the tube should pass freely once past the larynx and the horse may cough as the tube passes the carina.
- Continue passing the tube until resistance is felt and the tube bows as further pressure is applied, indicating a wedge has been formed.
- Inflate the cuff with around 5ml of air to form a seal.
- Instil 250ml to 500ml of warmed sterile saline, either as a single bolus or as two aliquots, and withdraw the fluid immediately. The fluid should be collected as rapidly as possible in case the seal around the tube is lost (Figure 3).
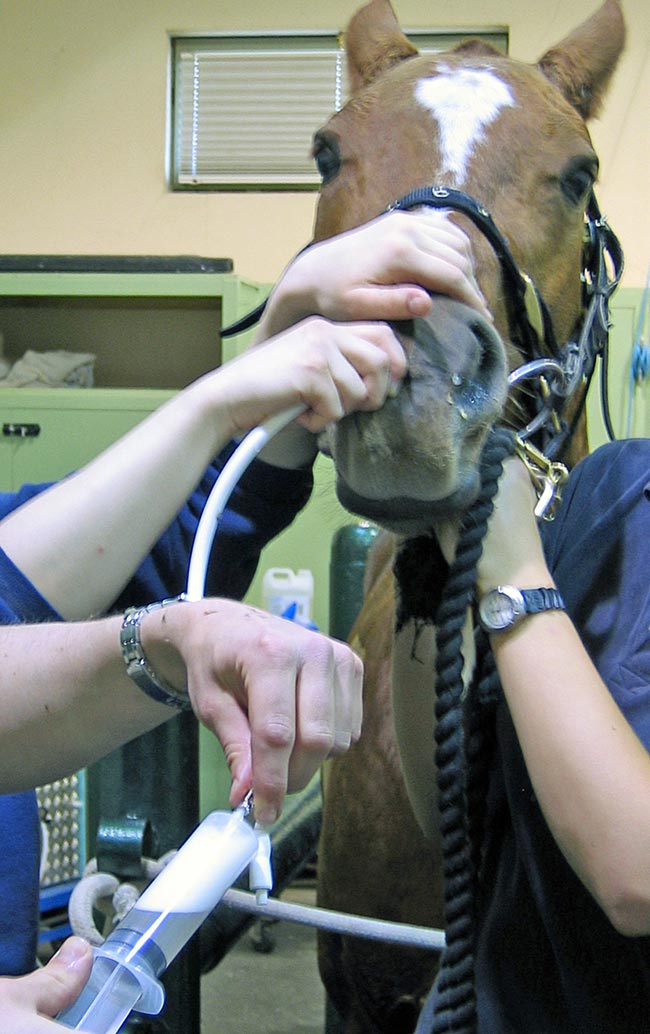
Figure 3. Examination of tracheal aspirate samples provides a subjective impression of the degree of airway inflammation. At least half of the instilled volume should be collected to ensure the resultant sample is representative of the distal airways, and not just of the area local to the tube tip. The sample should be foamy, indicating the presence of a surfactant and, hence, that adequate lavage of the alveoli has been performed (Hoffman, 2008; Figure 4).

Performing endoscopic BAL
- Pass the endoscope via the ventral nasal meatus into the trachea.
- Continue passing the endoscope past the carina until it wedges it in a distal bronchus forming a seal.
- Infuse 250ml to 500ml of warmed sterile saline via a catheter as fast as possible.
- Aspirate the sample.
- Discard the first 20ml and pool the remaining fluid prior to placing a sample into a container containing cytospin (or an EDTA blood tube) for cytological examination.
Sample submission
Samples for cytology should be chilled and analysed as soon as practical to minimise cell deterioration and bacterial growth. It is good practice to prepare, fix and send a smear concurrently to give the laboratory a reference for bacterial growth and sample degradation. Fluid samples should be chilled in transit (Pickles et al, 2002). Samples for bacteriology should not be placed in a preservative or fixative, but kept plain and transported chilled to the laboratory.
Analysing airway washes
Gross examination TAF and BALF
Gross examination of the sample provides further subjective information of the quantity of mucus present and, hence, an indication of airway inflammation (Figure 5). Normal samples will be clear or mildly grey and turbid. Increased turbidity or the presence of flocculent material indicates increased mucus and cellular material in the sample. Frank blood may be seen consistent with recent haemorrhage – typically as a result of exercise-induced pulmonary haemorrhage (EIPH) – or a brown sample may indicate the presence of less recent haemosiderin and haemorrhage. Plant material indicates contamination and a need for caution when interpreting the results of bacterial culture.

Cytology
White cell differential counts are the most important factor to consider when interpreting lower airway cytology. Absolute values are affected by dilution, which cannot be quantified and are, therefore, unhelpful. Epithelial cells should not be considered as part of the differential count.
Reference values for relative proportions of leukocytes in tracheal aspirate and bronchoalveolar lavage fluid are as follows:
| Cell type | TA (%) | BAL (%) |
| Macrophages | 40-80 | 40-70 |
| Lymphocytes | Less than 20 | 30-60 |
| Neutrophils | Less than 20 | Less than or equal to 5 |
| Eosinophils | Less than 1 | Less than 1 |
| Mast cells | Less than 1 | Less than 1 |
| (Hoffman, 2008; Richard et al, 2010; Couëtil et al, 2016). | ||
Macrophages
- The most abundant cell type in normal TAF and BALF samples.
- Commonly contain pollens, fungal spores and phagocytosed cell debris, even in the absence of respiratory disease.
- Haemosiderophages may be present for months after intense exercise in many racehorses. The presence of haemosiderophages does not necessarily indicate EIPH.
Lymphocytes
- A normal finding in BALF.
- Occur in low numbers in normal TAF.
- May be an indicator of a viral or other antigen challenge.
Neutrophils
-

Figure 4. Collection of a bronchoalveolar lavage sample using a nasobronchial tube. An increased proportion of neutrophils is the most consistent indicator of lower respiratory tract inflammation.
- Marked neutrophilia (more than 20%, often up to 90%) in BALF is seen with marked equine asthma and should not be taken as an indication of infectious challenge. Increases seen with allergic disease often exceed those seen with infectious disease.
- Variation in neutrophil proportions may be marked (up to 80%) in horses considered free of lower airway disease (Robinson et al, 2006), limiting the value of TAF cytology.
- Degenerate neutrophils are a feature of infectious disease, but may also be present in low numbers with non-infectious inflammatory disease.
- Lower airway neutrophilia is far more likely to be due to non-infectious disease and the presence of neutrophils (or mucus) is not an indication to use antimicrobials.
Eosinophils
- Presence of more than 80% to 90% eosinophils would be indicative of infection with Dictyocaulus arnfieldi (lungworm); however, this is rarely encountered.
- Mild eosinophilia is a feature of equine asthma.
Mast cells
- Rarely seen in TAF.
- Presence in BALF has been associated with reduced pulmonary compliance.
Mucus
- The quantity of mucus evident microscopically provides an indication of inflammation.
- In severe or chronic disease, mucus may form airway casts (Curschmann’s spirals).
Epithelial cells
- Epithelial cells should not be included as part of a differential count.
- Squamous epithelial cells should not be present in TAF and indicate oropharyngeal contamination. This contamination is inevitable when a BAL is performed and squamous epithelial cells may, therefore, be present in BALF.
- Columnar cells from the trachea and bronchi and cuboidal cells from the smaller airways are normal constituents of BALF.
Bacteria
- Extracellular bacteria are more likely to indicate contamination; intracellular bacteria are more likely to indicate infection.
- The presence of bacteria is only relevant if cytological evidence of inflammation exists – that is, neutrophilia and possibly the presence of degenerate neutrophils.
Bacteriology
Bacteriology should always be interpreted carefully, with consideration for contamination from collection – for example, from the end of the endoscope entering the fluid pool within the trachea or coughing during the procedure. Contamination is indicated by the presence of foreign material or oropharyngeal squamous epithelial cells (Figure 6). The presence of bacteria is considered significant if:
- clinical signs consistent with pneumonia or pleuropneumonia are present (such as pyrexia, tachypnoea, abnormal thoracic auscultation, lethargy or nasal discharge)
- isolates are known pathogens of the respiratory tract and are cultured in large numbers (more than 103 colony-forming units/ml)
- concurrent cytological evidence exists of inflammation (neutrophilia and increased mucus production)
- the sample is free of epithelial cells that would indicate contamination (Hodgson and Hodgson, 2007)
Bacterial species is important. Many species that are isolated commonly are known contaminants and their presence is not relevant clinically. Antimicrobial susceptibility should only be performed and acted on if the bacterial isolate is likely to be pathogenic. Bacteria commonly isolated from tracheal aspirates and their likely pathogenicity are as follows:
Likely pathogens
- Actinobacillus species
- Bordetella bronchiseptica
- Klebsiella pneumoniae
- Mycoplasma species
- Pasteurella species
- Rhodococcus equi
- Streptococcus dysgalactiae equisimilis
- Streptococcus equi equi
- Streptococcus pneumoniae
- Streptococcus equi zooepidemicus
Likely contaminants
- Escherichia coli
- Bacillus species
- Coliform
- Proteus species
- Pseudomonas species
- Staphylococcus species
- Alpha-haemolytic streptococci
- Enterococci species
(Source: Hodgson and Hodgson, 2007).
References
- Allen K and Franklin S (2007). RAO and IAD: respiratory disease in horses revisited, In Practice 29(2): 76-85.
- Cavanagh RL, Gylys JA and Bierwagen ME (1976). Antitussive properties of butorphanol, Arch Int Pharmacodyn Ther 220(2): 258-268.
- Christley RM, Hodgson DR, Rose RJ, Reid SWJ and Hodgson JL (1999). Comparison of bacteriology and cytology of tracheal fluid samples collected by percutaneous transtracheal aspiration or via an endoscope using a plugged, guarded catheter, Equine Vet J 31(3): 197-202.
- Cian F, Monti P and Durham A (2015). Cytology of the lower respiratory tract in horses: an updated review, Equine Vet Educ 27(10): 544-553.
- Couëtil LL, Cardwell JM, Gerber V, Lavoie JP, Léguillette R and Richard EA (2016). Inflammatory airway disease of horses – revised consensus statement, J Vet Intern Med 30(2): 503-515.
- Fraipont A, van Erck E, Ramery E, Richard E, Denoix JM, Lekeux P and Art T (2011). Subclinical diseases underlying poor performance in endurance horses: diagnostic methods and predictive tests, Vet Rec 169(6): 154.
- Gerber V, Robinson NE, Luethi S, Marti E, Wampfler B and Straub R (2003). Airway inflammation and mucus in two age groups of asymptomatic well-performing sport horses, Equine Vet J 35(5): 491-495.
- Gerber V, Straub R, Marti E, Hauptman J, Herholz C, King M, Imhof A, Tahon L and Robinson NE (2004). Endoscopic scoring of mucus quantity and quality: observer and horse variance and relationship to inflammation, mucus viscoelasticity and volume, Equine Vet J 36(7): 576-582.
- Hodgson JL and Hodgson DR (2007). Collection and analysis of respiratory tract samples. In McGorum BC, Dixon PM, Robinson NE and Schumacher J (eds), Equine Respiratory Medicine and Surgery, WB Saunders, Edinburgh: 119-150.
- Hoffman AM (2008). Bronchoalveolar lavage: sampling technique and guidelines for cytologic preparation and interpretation, Vet Clin North Am Equine Pract 24(2): 423-435.
- Hotchkiss JW, Reid SW and Christley RM (2007). A survey of horse owners in Great Britain regarding horses in their care, part 2: risk factors for recurrent airway obstruction, Equine Vet J 39(4): 301-308.
- Kutasi O, Balogh N, Lajos Z, Nagy K and Szenci O (2011). Diagnostic approaches for the assessment of equine chronic pulmonary disorders, J Equine Vet Sci 31(7): 400-410.
- Larson VL and Busch RH (1985). Equine tracheobronchial lavage: comparison of lavage cytologic and pulmonary histopathologic findings, Am J Vet Res 46(1): 144-146.
- Mazan MR (2015). Update on noninfectious inflammatory diseases of the lower airway, Vet Clin North Am Equine Pract 31(1): 159-185.
- Pickles K, Pirie RS, Rhind S, Dixon PM and McGorum BC (2002). Cytological analysis of equine bronchoalveolar lavage fluid, part 3: the effect of time, temperature and fixatives, Equine Vet J 34(3): 297-301.
- Richard EA, Fortier GD, Lekeux PM and Erck EV (2010). Laboratory findings in respiratory fluids of the poorly-performing horse, Vet J 185(2): 115-122.
- Robinson NE, Karmaus W, Holcombe SJ, Carr EA and Derksen FJ (2006). Airway inflammation in Michigan pleasure horses: prevalence and risk factors, Equine Vet J 38(4): 293-299.
- Slater J (2014). Equine disease surveillance, Vet Rec 175(11): 271-272.
- Wasko AJ, Barkema HW, Nicol J, Fernandez N, Logie N and Léguillette R (2011). Evaluation of a risk-screening questionnaire to detect equine lung inflammation: results of a large field study, Equine Vet J 43(2): 145-152.
- Winder NC, Gruenig G, Hermann M, Howald B and von Fellenberg R (1989). Comparison of respiratory secretion cytology and pulmonary histology in horses, J Vet Med 36(1): 32-38.
