21 Jan 2021
Laminitis symptoms and treatment, and helping owners spot early signs
Nicola Menzies-Gow discusses the need for targeted owner education to raise awareness of clinical signs associated with this common issue.

Image: Mari_art / Adobe Stock
Laminitis is now considered to be a clinical syndrome associated with systemic disease (sepsis or systemic inflammatory response syndrome, or endocrine disease) or altered weightbearing, rather than being a discrete disease entity. Three forms of laminitis exist – sepsis-associated, endocrinopathic and supporting limb.
A wide range of clinical signs are displayed by laminitic cases and no individual – or combination of – clinical signs are present in every case. The signs that have been shown to be the most useful when trying to distinguish laminitis from other lameness causes are the presence of “reluctance to walk”, “short, stilted gait at walk”, “difficulty turning”, “shifting weight” and an “increased digital pulse”.
Owners recognise laminitis in approximately half of cases that are subsequently diagnosed as laminitis by a vet. Undefined lameness, foot abscesses, colic and stiffness are common reasons for owner-requested veterinary visits in owner-unrecognised cases. Therefore, further targeted owner education is required to raise awareness of the clinical signs that have been shown to be most commonly associated with laminitis, rather than those commonly perceived to be present. Providing owners with a list of potential clinical signs to be aware of, including questions relating to management and clinical history of their animals, could encourage more rapid and proactive decision-making.
Treatment of laminitis consists of providing foot support and adequate analgesia, as well as treating any underlying condition.
Laminitis is now considered to be a clinical syndrome associated with systemic disease (sepsis or systemic inflammatory response syndrome [SIRS], or endocrine disease) or altered weightbearing, rather than being a discrete disease entity.
Three forms of laminitis exist:
- Sepsis-associated laminitis occurs secondary to SIRS and/or sepsis, and so occurs in animals with, for example, severe gastrointestinal disease, pleuropneumonia and septic metritis following retention of fetal membranes.
- Endocrinopathic laminitis is the most common form of laminitis, accounting for 90% of cases of it in some studies1. It includes laminitis associated with insulin dysregulation, as occurs in equine metabolic syndrome (EMS), and in a subset of animals with pituitary pars intermedia dysfunction (PPID).
- Supporting limb laminitis (SLL) is uncommon. However, it is a major contributor to treatment failure in painful limb conditions such as fractures and refractory cases of synovial sepsis, as laminitis develops in the contralateral limb.
Symptoms of laminitis
The symptoms of laminitis are the same regardless of type and include any combination of:
- lameness that usually affects two or more limbs, but lameness in one limb is possible
- reluctance to walk with a short, stilted gait and difficulty turning
- increased (or bounding) digital pulses
- increased hoof wall temperature
- weight shifting
- pain on hoof tester pressure at the toe region just in front of the point of the frog
- characteristic stance of leaning back on the heels and taking weight off the painful toe region
- palpable depression at the coronary band
The lameness can vary in severity – from that which is only perceptible at the trot, through to spending prolonged periods recumbent.
It should be remembered some episodes of laminitis are subclinical and while no overt lameness (or any other clinical sign) may exist, it subsequently becomes apparent as divergent hoof growth rings.
Helping owners to spot early signs
A recent study sought to establish the extent owners were able to recognise laminitis, and the basis on which they did so2.
Of the 93 cases of veterinary-diagnosed active laminitis included in the study, 51 (54.8%) had been suspected as having laminitis by their owners. All 51 of these owner‑suspected cases of laminitis were confirmed by a veterinary surgeon – no “false positive” cases of owner‑suspected laminitis were reported. Nearly 80% of the owners who suspected laminitis, which was also subsequently diagnosed by a veterinary surgeon, had previous direct experience with the disease.
The 42 (45.2%) owners who did not suspect laminitis in their animals either did not know what the problem was, or suspected another lameness condition (foot abscesses, bruised sole or navicular disease) or another condition (colic, musculoskeletal stiffness, sunburned heels and swollen sheath).
When the clinical signs associated with laminitis reported by the owner and attending veterinarian for the same horse were compared, consistency existed for the reported presence of most clinical signs, underlying conditions and risk factors associated with laminitis. However, significant differences existed for four clinical signs – difficulty turning and weight shifting were more frequently reported by vets, while increased hoof temperature and recumbency were reported more frequently by owners2.
Additionally, owners were less likely than vets to report EMS as an underlying condition and less likely to recognise laminitis in non-pony breeds.
A second study aimed to compare the prevalence of selected clinical signs in laminitis and non-laminitis lameness cases to evaluate the capabilities of clinical signs to differentially diagnose laminitis from other causes of lameness3.
A wide range of clinical signs were displayed by the laminitic cases and no individual – or combinations of – clinical signs were present in every case. The clinical signs that were considered to be the most useful were three features of lameness investigation (“reluctance to walk”, “short, stilted gait at walk” and “difficulty turning”), one feature of stance (“shifting weight”) and an “increased digital pulse”. All these signs had a difference in prevalence of greater than 50% between active laminitis cases (signs more prevalent) and non-laminitic lame horses (signs less prevalent).
Two clinical signs – “coronary band depression” and “prolapsed sole” – that are considered pathognomonic for laminitis were only found in 13.6% and 3.7% of cases, respectively. Additionally, “front feet in front of the body” – taken to represent the classic “laminitis stance” – was found in less than half of the diagnosed active laminitis cases, and did not prove to be a useful discriminator.
Therefore, despite much anecdotal publicity of this visibly apparent clinical sign, veterinarians and owners should be careful to avoid relying on its presence for making a diagnosis of laminitis.
Two overall combined trees were generated to reflect the two clinical scenarios of active laminitis – one consisting of clinical signs considered to occur in the acute phase of the disease (Figure 1), and one that also contained data reflective of lamellar damage and displacement of the pedal bone (Figure 2).
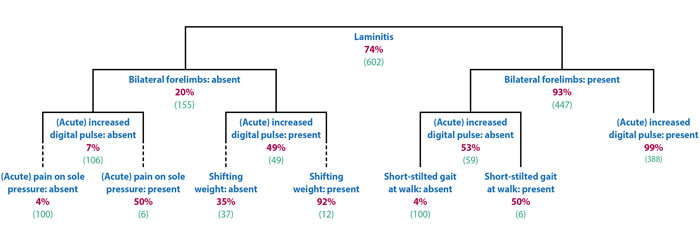
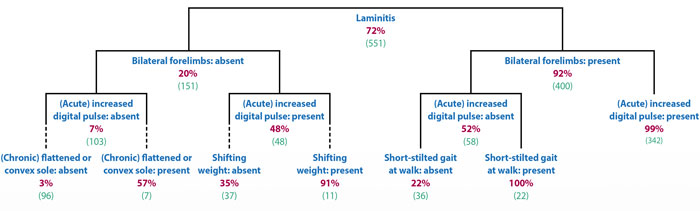
In both scenarios, the presence of a bilateral lameness was the most useful discriminator, followed by the presence of increased digital pulses.
Therefore, further targeted owner education is required to raise awareness of the clinical signs that have actually been shown to be most commonly associated with laminitis, rather than those commonly perceived to be present.
Providing owners with a list of potential clinical signs to be aware of – including questions relating to management and clinical history of their animals – could encourage more rapid and proactive decision‑making.
Owner education could further be targeted to owners lacking previous direct experience of the disease and those owning breeds not perceived to be at risk.
Treatment
Treatment involves providing analgesia and foot support, as well as treating the underlying endocrinopathy or primary condition causing sepsis-associated laminitis or SLL. Additionally, cryotherapy is indicated in certain circumstances.
- Some drugs mentioned in this article are used under the cascade.
References
- Karikoski NP, Horn I, McGowan TW and McGowan CM (2011). The prevalence of endocrinopathic laminitis among horses presented for laminitis at a first-opinion/referral equine hospital, Domest Animal Endocrinol 41(3): 111-117.
- Pollard D, Wylie CE, Verheyen KLP and Newton JR (2017). Assessment of horse owners’ ability to recognise equine laminitis: a cross-sectional study of 93 veterinary diagnosed cases in Great Britain, Equine Vet J 49(6): 759-766.
- Wylie CE, Shaw DJ, Verheyen KL and Newton JR (2016). Decision-tree analysis of clinical data to aid diagnostic reasoning for equine laminitis: a cross-sectional study, Vet Rec 178(17): 420.
- Menzies-Gow NJ, Stevens K, Barr A et al (2010). Severity and outcome of equine pasture-associated laminitis managed in first opinion practice in the UK, Vet Rec 167(10): 364-369.
- West E, Bardell D, Morgan R and Senior M (2011). Use of acetaminophen (paracetamol) as a short-term adjunctive analgesic in a laminitic pony, Vet Anaesth Analg 38(5): 521-522.
- Thomasy SM, Slovis N, Maxwell LK and Kollias-Baker C (2004). Transdermal fentanyl combined with nonsteroidal anti-inflammatory drugs for analgesia in horses, J Vet Intern Med 18(4): 550-554.
- Orsini JA, Moate PJ, Kuersten K et al (2006). Pharmacokinetics of fentanyl delivered transdermally in healthy adult horses – variability among horses and its clinical implications, J Vet Pharmacol Ther 29(6): 539-546.
- Guedes A, Knych H and Hood D (2016). Plasma concentrations, analgesic and physiological assessments in horses with chronic laminitis treated with two doses of oral tramadol, Equine Vet J 48(4): 528-531.
- Stewart AJ, Boothe DM, Cruz-Espindola C et al (2011). Pharmacokinetics of tramadol and metabolites O-desmethyltramadol and N-desmethyltramadol in adult horses, Am J Vet Res 72(7): 967-974.
- Dhanjal JK, Wilson DV, Robinson E et al (2009). Intravenous tramadol: effects, nociceptive properties, and pharmacokinetics in horses, Vet Anaesth Analg 36(6): 581-590.
- Guedes AGP, Matthews NS and Hood DM (2012). Effect of ketamine hydrochloride on the analgesic effects of tramadol hydrochloride in horses with signs of chronic laminitis-associated pain, American Journal of Veterinary Research 73(5): 610-619.
- Davis JL, Posner LP and Elce Y (2007). Gabapentin for the treatment of neuropathic pain in a pregnant horse, J Am Vet Med Assoc 231(5): 755-758.
- Terry RL, McDonnell SM, Van Eps AW et al (2010). Pharmacokinetic profile and behavioral effects of gabapentin in the horse, J Vet Pharmacol Ther 33(5): 485-494.
- Guedes AGP, Morisseau C, Sole A et al (2013). Use of a soluble epoxide hydrolase inhibitor as an adjunctive analgesic in a horse with laminitis, Vet Anaesth Analg 40(4): 440-448.
- van Eps AW, Pollitt CC, Underwood C et al (2014). Continuous digital hypothermia initiated after the onset of lameness prevents lamellar failure in the oligofructose laminitis model, Equine Vet J 46(5): 625-630.
- Kullmann A, Holcombe SJ, Hurcombe SD et al (2014). Prophylactic digital cryotherapy is associated with decreased incidence of laminitis in horses diagnosed with colitis, Equine Vet J 46(5): 554-559.
- Stokes SM, Belknap JK, Engiles JB et al (2019). Continuous digital hypothermia prevents lamellar failure in the euglycaemic hyperinsulinaemic clamp model of equine laminitis, Equine Vet J 51(5): 658-664.
- Longland AC, Barfoot C and Harris PA (2011). Effects of soaking on the water-soluble carbohydrate and crude protein content of hay, Vet Rec 168(23): 618.
