13 Sept 2019
David Rendle discusses the endoparasites associated with this common presentation and how good management strategies should ensure infrequent use of anthelmintics aimed at prevention.
Gastrointestinal parasites are – and should be – present in all horses.
In the past, we have made the mistake of trying to eliminate parasites that have the potential to cause disease, particularly colic. We failed to eliminate these parasites, and the unintended consequence of overzealous use of anthelmintics was anthelmintic resistance such that we now face the threat of disease as a result of infestation with parasites we are unable to kill.
Interval dosing with anthelmintics was introduced in the 1960s to prevent colic associated with Strongylus vulgaris infection. In the 1990s, cyathostomin resistance became a concern and it was recommended interval dosing should be discontinued in favour of more targeted parasite control strategies that reduced selection pressure.
Change has been slow, and these recommendations are still frequently ignored. Morbidity and mortality associated with the presence of resistant cyathostomins and ascarids is now relatively frequent, and colic is a common presenting sign. Signs of resistance are now developing in tapeworms1 and it would be surprising if resistance was not present among large strongyles2.
The endoparasites most likely to be associated with colic in adult horses in temperate climates are Anoplocephala perfoliata and S vulgaris, the latter being extremely rare. Cyathostomins are not typically associated with colic, but the intestinal pathology they incite can be associated with abdominal discomfort accompanied by other clinical signs. On rare occasions, aberrant larval migrans and other parasites may also cause signs of abdominal discomfort.
In young foals, Strongyloides westeri is an uncommon cause of enteritis that may occasionally result in colic. Parascaris equorum is a relatively common cause of colic in foals and weanlings.
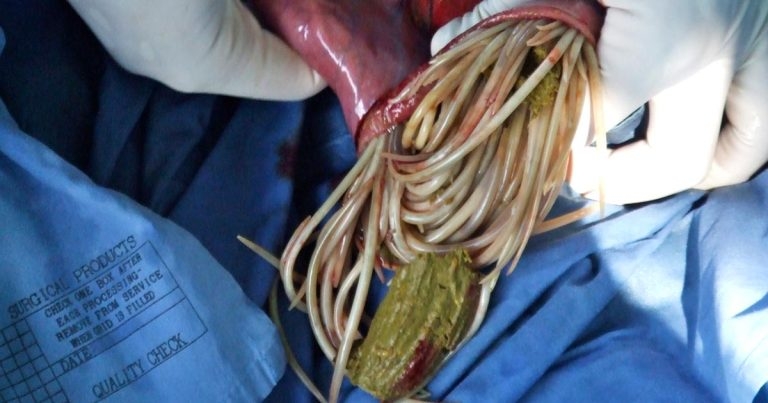
Three species of anoplocephalid cestodes are known to infect horses: A perfoliata, Anoplocephala magna and Anoplocephaloides mamillana (formerly known as Paranoplocephala mamillana).
A perfoliata is by far the most prevalent, with the other two species rarely reported. Reports of colic associated with tapeworm infection have all been attributed to A perfoliata infection.
A mamillana and A magna inhabit the small intestine, while A perfoliata typically inhabits the caecum near the ileocaecal junction.
Oribatid mites are essential intermediate hosts and ubiquitous in the environment; their movement may also play a role in disseminating infection. The life cycle of A perfoliata is such that higher burdens and patent infection are more likely to develop in the second half of the year, following ingestion during the grazing season.
A perfoliata results in localised inflammation and mucosal thickening around the site of attachment, and an association exists between the intensity of infection and severity of pathology3. A number of case reports and case control studies have identified an association between tapeworm infestation and colic, with the risk of colic increasing in horses that have tapeworm infestation. Tapeworm infection has been associated with spasmodic colic, ileal impaction, ileal hypertrophy, ileal rupture, intussusception, caecal perforation, caecal torsion and abscessation, and a higher frequency of colic has been reported in populations of young horses that have not been treated4.
Outbreaks of colic have been identified in young horses when no anticestode treatment has been administered5. However, not all studies have identified a relationship, the majority of horses infected with tapeworms do not develop colic and the actual increase in risk of colic in an individual horse has not been established4.
On balance, A perfoliata infection probably does result in colic under certain circumstances, but the aetiology may be multifactorial and the actual risk needs to be balanced against the negative impact on the population of indiscriminate use of cestodicidal drugs.
In abattoir studies performed in the UK, prevalence of infection is around 50%6-8, with only about 25% having a level of infection that may be relevant clinically. Given the low prevalence of infection – and relatively low risk of disease with low infection intensity – blanket treatment of tapeworms should be considered obsolete, and horses with a high level of infection should be identified and treatment targeted at those animals.
Diagnosis of tapeworm infection was traditionally performed by faecal analysis to detect the presence of cestode eggs, but this method has fallen out of favour because it appeared to be an insensitive technique for detecting the presence of tapeworms. The intermittent shedding of tapeworm eggs – as egg-containing segments are periodically shed – may compromise the effectiveness of the technique. However, as our focus moves away from detecting the presence of tapeworms, per se, to differentiating horses that need treatment from those that don’t, the benchmark for any test is moved.
Faecal analysis using sedimentation-flotation methods optimised for detection of tapeworm eggs – rather than the methods routinely used for the detection of cyathostomin eggs – has been shown to be 90% reliable in identifying a tapeworm burden of 20 or more worms9,10, which is regarded as the level at which disease is more likely to occur3. Faecal analysis is, therefore, in the author’s opinion, a valid and underused means of identifying the presence of a level of infection that may increase the risk of colic. By contrast to the measurement of antibody levels, detection of eggs in faeces can differentiate current from recent (up to six months) infection.
Cestode eggs are more likely to be released after the administration of treatments effective against tapeworms11, so faecal analysis performed at this time can be helpful in confirming the presence of tapeworms that might have been relevant to an episode of colic.
Measurement of antibody levels in serum or saliva is more straightforward than faecal analysis and, therefore, a more popular means of assessing levels of infection.
Both serum and saliva methods have been demonstrated to be reliable, but both have the limitation that they cannot differentiate current from previous infection, as it takes a number of months for antibody levels to wane. For the serum test, it takes up to five months for antibody levels to return to normal12; for the saliva test, levels should normalise within three months6.
Depending on the level of risk – for example, age, history of colic, history of parasite control on the yard, stocking density and horse movement – measurement of antibody levels is recommended once or twice per year. In most situations, annual testing and treatment is likely to be sufficient. With biannual monitoring, a risk exists of reduced compliance as a result of the greater cost.
Despite warnings from anthelmintic resistance in small strongyles, blanket treatment of tapeworms to reduce the risk of colic is still common practice. Application of a targeted treatment plan for tapeworms – using the saliva test – has been shown to reduce the requirement for anthelmintics by 86%13, the same reduction that occurs with targeted treatment for cyathostomin control. The cost of the serological or saliva test is often cited as a barrier to their use; however, the reduction in anthelmintic use by more than 80% goes some way to offsetting the expense of testing.
The presence of antibodies against A perfoliata does not indicate a need to treat and the interpretation of the blood test has been revised in recent years such that it is used to identify horses with a level of antibodies indicative of a burden sufficient to cause disease10. The interpretation of both serum and saliva tests remains conservative, and is still likely to result in over-treatment to ensure horses at potential risk of colic do not go untreated.
In response to a high antibody result, treatment with pyrantel or praziquantel can be administered. Praziquantel is preferred, as it is a specific anticestode treatment and avoids placing a selection pressure against other helminths. The licensed product has been withdrawn, but praziquantel is available as a special that can be used in accordance with the cascade.
Pyrantel will have activity against nematodes as well as cestodes, which may be advantageous if a high faecal cyathostomin egg count exists; however, if it does not then praziquantel would be the preferred option.
When used against cestodes, pyrantel is used at 13.2mg/kg (as base), twice the dose it is used at against nematodes (6.6mg/kg as base). The nematode dose has been demonstrated to remove more than 60% of cestodes14, which, in most horses, is likely to be enough to prevent tapeworm-related disease.
If treatment is being targeted against tapeworms then pyrantel should be used at the higher licensed dose; however, if a horse with a high antibody titre has received treatment with pyrantel at 6.6mg/kg within the past few months, further treatment may not be necessary.
As levels of tapeworm infestation appear to increase through the grazing season, testing and treatment in autumn/winter is logical when it is being performed on an annual basis.
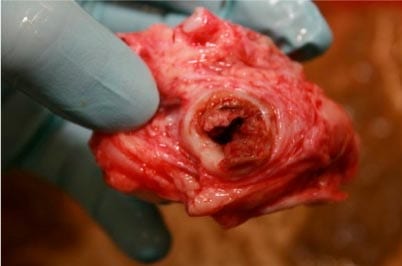
Veruminous arteritis caused by the large strongyle S vulgaris was a well-recognised cause of intestinal ischaemia – and, therefore, colic – until the use of macrocyclic lactones became routine. Reports exist of re-emergence in Denmark, where the use of anthelmintics has been restricted to slow the development of anthelmintic resistance2.
If they are present in sufficient numbers, larval stages may cause arteritis and thrombosis, which typically develops at the root of the cranial mesenteric artery (Figure 1). Antemortem diagnosis is extremely challenging, with diagnosis generally made during exploratory laparotomy or at postmortem examination after presentation with signs of acute colic and intestinal ischaemia.
Horses that develop ischaemic lesions are typically infected with hundreds of larvae, and the life cycle takes between six and seven months to complete. Infective third-stage larvae are ingested from the environment, invade the submucosa of the small intestine and moult to fourth-stage larvae.
Fourth-stage larvae penetrate arterioles, burrow beneath the intima and migrate proximally, where most of them accumulate at the root of the cranial mesenteric artery. Fourth-stage larvae reside there for about four months and moult to fifth-stage larvae, which enter the bloodstream and circulate to the caecum, where they form nodules in the submucosa and develop into adults. The nodules ultimately rupture – releasing adults into the lumen of the caecum, which, after a few weeks of maturation, become sexually mature and release eggs into the lumen to be excreted in faeces.
S vulgaris is sensitive to macrocyclic lactones; therefore, specific treatment is unlikely to be required unless no other anthelmintics are being administered for a year or more. The parasite cannot survive for more than a year in the environment and has probably been eradicated on most properties where anthelmintics have been used regularly for years or decades. We should, however, remain cognisant of the risk of disease – particularly as horses that have never been exposed to the parasite may have little immunity to it.
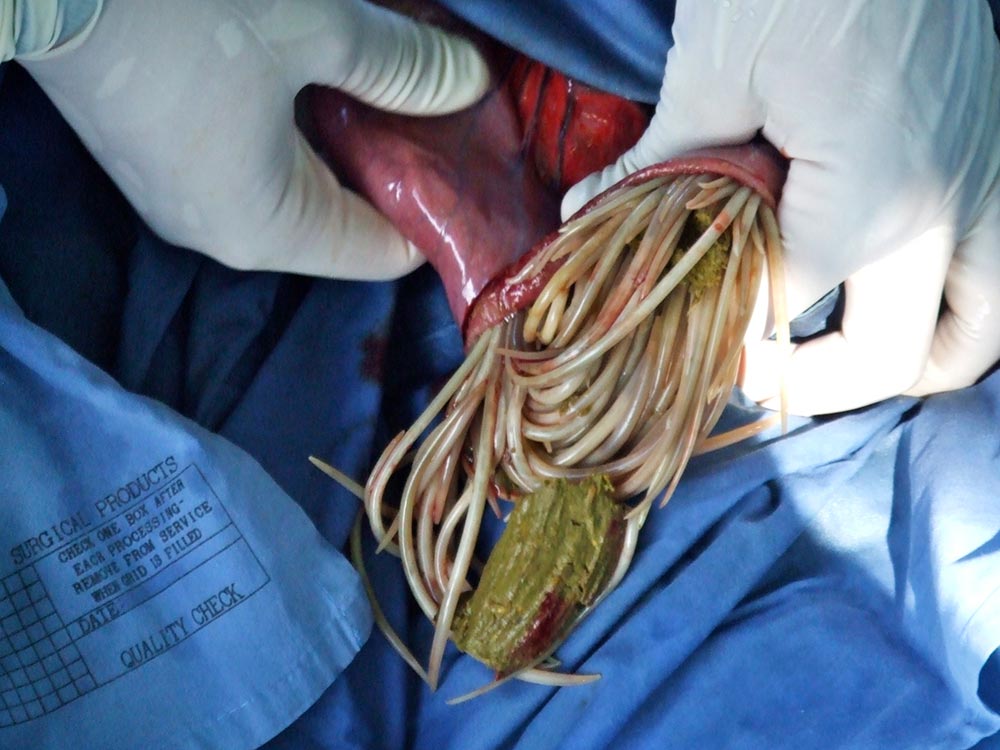
Parascaris equorum is an increasingly important cause of colic in foals as resistance to multiple classes of anthelmintic becomes more widespread. Adult ascarids may be up to 25cm long and 4mm in diameter2, and in large numbers may obstruct the small intestine – resulting in colic (Figure 2).
Clinical signs often follow the administration of anthelmintic and obstruction with a entangled ball of dead parasites. Foals present with evidence of small intestinal obstruction – and on ultrasonography, parasites are often visible as linear hyperechoic structures within the small intestine that are dilated with anechoic fluid. Clinical signs are most likely to occur in late summer and autumn. Signs of respiratory disease may also be seen in younger foals as a result of larval migration.
Some ascarid impactions may respond to medical treatment; however, cases that present with complete obstruction of the small intestine frequently require surgery to remove the parasites. Anthelmintics have to be administered at some point, but doing so risks worsening any obstruction. Supportive and postoperative care are required, as for any case with small intestinal obstruction.
Larvated eggs are ingested from pasture, hatch within the intestine and migrate to the liver via the portal circulation. Larvae re-enter the circulation after about a week and travel to the lungs – where they migrate into the alveoli, up the airways, and are swallowed.
Larvae moult into adults within the small intestine, with eggs being excreted in faeces approximately three months after infection. Most horses will develop a robust acquired immune response to P equorum and infection is uncommon in horses aged two years or older.
Resistance of P equorum to macrocyclic lactones is now common – ubiquitous in some areas – and resistance to pyrantel and fenbendazole is increasingly observed1. Therefore, faecal egg count reduction tests are essential in preventing and managing ascarid infection. Regular faecal worm egg counts should be used to determine the need for anthelmintic administration, to control the risk of disease associated with P equorum, and limit buildup of infective stages on pasture.
Pasture management is critical on properties with large numbers of foals1.
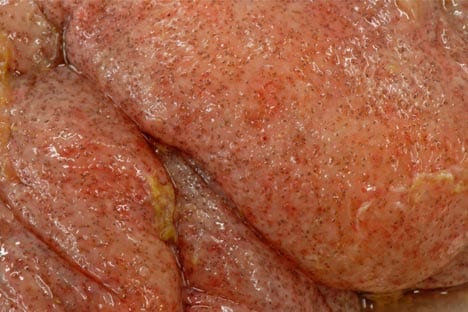

Cyathostomins are not generally considered to be a cause of colic; however, colic signs may be seen in association with larval cyathostominosis, and the colonic oedema and inflammation it causes (Figure 3). Indicators other than colic always exist that alert clinicians to the possibility of cyathostominosis, including weight loss, ventral oedema, diarrhoea, hypoalbuminaemia and lethargy (Figure 4).
Prevention should comprise regular monitoring of faecal worm egg counts in the population through the grazing season, to limit pasture contamination and risk of infection. In the autumn, risk of infection with larval cyathostomins should be assessed on an individual horse basis, with treatment administered accordingly.
In most horses, moxidectin should be used as it has the greatest efficacy against larval cyathostomins. Moxidectin should be reserved for this purpose and avoided at other times of year to reduce selection pressure. Five days of fenbendazole is also licensed for the treatment of larval cyathostomins, but resistance is now so common it should not be used in the absence of testing to demonstrate efficacy.
Removal of encysted larval cyathostomins in horses with clinical cyathostominosis can be challenging – and once clinical disease develops, prognosis is poor.
Treatment regimens have been invented by clinicians and have little evidence base. The author administers moxidectin at two-week to four-week intervals, until a trend of increasing albumin concentration is present. A perception exists that fenbendazole may be safer, as it elicits less of an inflammatory response. This belief is not supported by recent work – indeed, evidence exists to the contrary15. Given almost ubiquitous resistance in the UK, fenbendazole is unlikely to be effective.
Twenty-four hours prior to the administration of the first dose of moxidectin, the author will often administer dexamethasone (0.1mg/kg IV) and continue treatment with dexamethasone or prednisolone (1mg/kg to 2mg/kg) until albumin concentrations are improving.
If albumin is low (lower than 20g/L) then plasma administration should be considered. Synthetic colloids, such as hetastarch, may also help raise colloidal oncotic pressure and can be used additionally to plasma or as an alternative.
Crystalloids are overused in the treatment of cyathostominosis and may worsen prognosis by potentiating intestinal oedema.
Most horses with cyathostominosis have chronic disease and do not have marked fluid deficits. If isotonic fluids are required, they can often be administered orally with other treatments by nasogastric tube.
Additional supportive treatments may include codeine to reduce fluid losses, analgesics (NSAIDs and/or opioids), a digestive supplement or other absorbents to bind faecal contents (and possibly absorb toxins), and misoprostol. Nursing care to manage oedema and pressure sores, for example, and encourage eating is important.
Cohorts of any horse with larval cyathostominosis should be examined and have serum albumin concentration measured to identify horses with subclinical disease.

Stongyloides infection is not uncommon in nursing foals, but is a rare cause of enteritis and colic (Figure 5). Diarrhoea may occur in the absence of colic.
Larvae are present within the mare and migrate to the mammary glands during pregnancy, from where they are ingested by the foal within the first week of lactation. Larvae develop into patent adults within the small intestine within a couple of weeks.
S westeri is very sensitive to ivermectin, and treatment should only be administered in response to clinical signs as the risk of disease is low and preventive treatment is not necessary.
It is clear a balance needs to be struck between preventing colic and other parasitic disease, while minimising the selection pressure for resistance.
While the risk of colic associated with endoparasitism remains real, in the author’s opinion, the risks are frequently overstated. The scales are tipped heavily in favour of preventing disease – and to maintain the health of the population in the future, they need to level up, if not tip slightly in the other direction.
With good management, it should be possible to avoid having to make a choice and use anthelmintics very infrequently without compromising the health of individual animals.