27 Feb 2024
Performance: what are the treatment options?
Mélanie Perrier looks at the different choices on offer including biologics, meds, bisphosphonates and NSAIDs, including COX selectivity.

Image © Valeri Vatel / Adobe Stock

When evaluating performance in horses, the most common limiting factor is musculoskeletal dysfunction followed by respiratory and cardiovascular disease. To ensure an optimal response to treatment, an appropriate and complete diagnosis must be made, as in a number of cases multiple conditions may be present.
Once this is obtained, a treatment plan can be put in place and in most cases the first and main goal will be to control inflammation, attenuate pain and restore function. In many cases, this will be at least in part achieved by using systemic medication but also in some cases local therapy. This article provides a general overview of some of the products available and their advantages and disadvantages.
NSAIDs
Non-steroidal anti-inflammatory medication remains the most common first line of treatment aiming at reducing inflammation and providing analgesia1. NSAIDs represent a class of drugs that inhibit one or more pathways involved with the synthesis of prostaglandins (PGs) and thromboxanes (TBXs) from arachidonic acid.
The principal mechanism of action is through inhibition of the enzyme cyclooxygenase (COX). There are two main cyclooxygenase: COX-1 and COX-2; the COX-1 isomer is mainly responsible for beneficial physiological functions while COX-2 is believed to mediate detrimental effects, particularly in regards to renal blood flow and gastrointestinal integrity. Most NSAIDs are competitive COX agonist and the COX-2/COX-1 ratio can be used to reflect the relative selectivity of an NSAID for a specific COX isomers2,3.
Firocoxib is the only drug available on the market that is labelled for horses and is COX-2 selective4. Meloxicam is mostly COX-2 selective and is also labelled for used in horses in Europe5. The other commonly used drugs have a variability of affinity for the different COX isoforms and slightly different mechanism of action which may guide their choice for specific clinical requirements. For example, aspirin is an irreversible antagonist that deactivates COX by acetylation. Because platelets are nonnucleated and do not have the capacity to synthesise new COX, aspirin has a profound effect on platelet function and may be best indicated for thrombophlebitis treatment. Please refer to Table 1 for a list of commonly used NSAIDs and their mechanism of action and indications.
| Table 1. A list of commonly used NSAIDs and their mechanism of action and indications | |||||
|---|---|---|---|---|---|
| Drug | Administration | Dose | Action | Indication | Indicative detection time (hr) |
| Aspirin | PO | 25mg/kg to 35mg/kg bid 5mg/kg to 50mg/kg sid |
Irreversible inhibition of platelet cyclooxygenase (COX; acetylation) | Thrombophlebitis, intestinal ischemia and non-strangulating infarction | 48 (2) |
| Dipyrone | IV, IM | 11mg/kg qid | Inhibits endogenous PGE2, raises threshold level of thermoregulatory centre in hypothalamus | Anti-pyretic | 72 (3d) |
| Firocoxib | PO | 0.1mg/kg sid | COX-2 selective drug | 336 (6d) | |
| Flunixin | PO, IV | 1.1mg/kg sid to bid | Visceral analgesia Anti-endotoxic effects |
144 (6d) | |
| Ketoprofen | IV | 2.2mg/kg sid | Decreases synovial fluid PGE2 concentrations | Pain associated with musculoskeletal disease and colic Joint effusion, synovitis Hoof pain |
96 (4d) |
| Phenylbutazone | PO, IV | 2.2-4.4 mg/kg sid to bid | Musculoskeletal analgesia Reducing surgical wound oedema |
168 (7d) | |
| Carprofen | PO, IV | 0.7mg/kg sid | Decreases inflammatory exudate PGE2 levels and ex-vivo TXB2 generation | Decreasing inflammation associated oedema and joint effusion | |
| Meloxicam | PO, IV | 0.6mk/kg sid | Mostly COX-2 selective | Decrease inflammation Analgesia |
73 (3d) |
| PO = by mouth, sid = once a day, bid = twice a day, qid = four times a day. | |||||
Corticosteroids
Corticosteroids are also frequently used for their anti-inflammatory and analgesic properties and have the advantages that they can be used both systemically and locally. They remain, to date, the most common drug used for intraarticular therapy in horses. Glucocorticoids exert their anti-inflammatory effects slightly differently from NSAIDs (see diagram) as they stimulate inhibition of the activity of plasma membrane-bound phospholipase A2 (PLA2) by stimulation of lipocortin and thereby inhibits release of arachidonic acid; this, in turn, will alter the synthesis of inflammatory mediators such as PGs, TBXs, LTs, and platelet activating factors6 (see diagram).
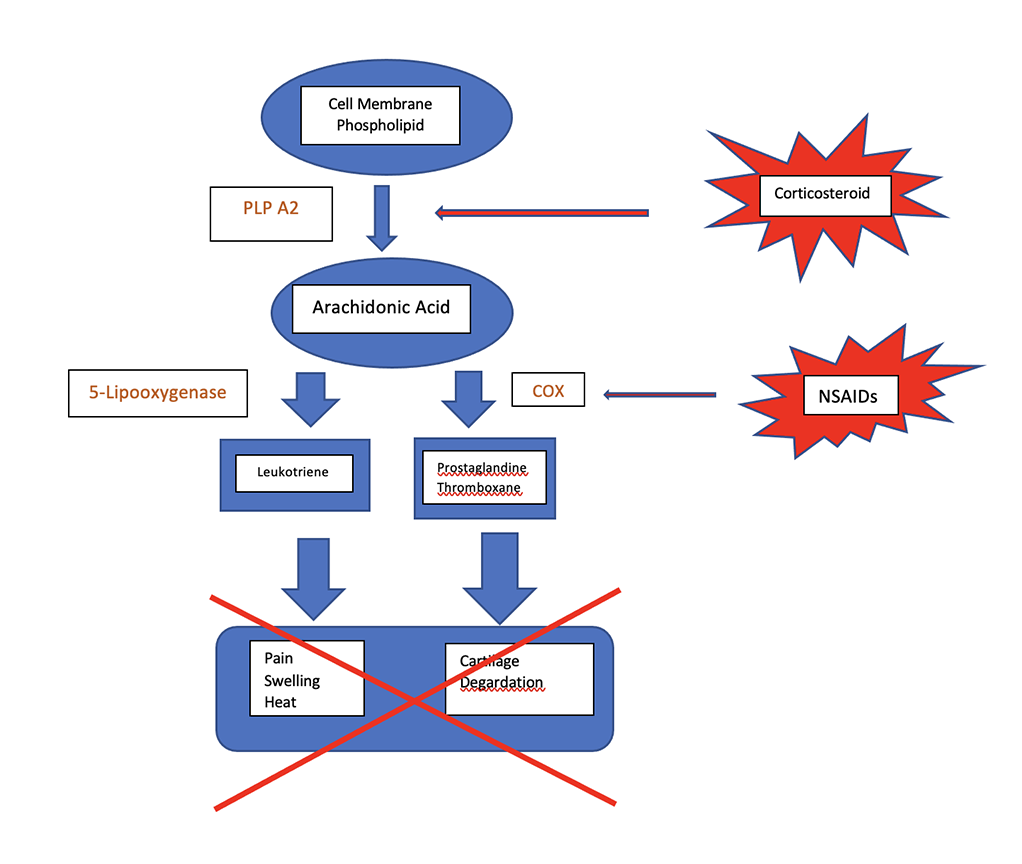 The most common used steroids in equine practice are triamcinolone, methylprednisolone and betamethasone. The main difference is their duration of action. Some steroids may be preferred by some clinicians depending on their own characteristics: betamethasone has no reported deleterious side-effects, triamcinolone has been shown in some study to have some chondroprotective action and may promote cartilage health. On the other hand, methylprednisolone has been shown to have deleterious effects on cartilage in some studies and therefore is preferred by most clinicians for use in low motion joints such as the tarsometatarsal joint. There is some scientific evidence that hyaluronic acid (HA) exhibits some chondroprotective effects long-term and therefore combination therapy with triamcinolone or betamethasone may be appropriate.
The most common used steroids in equine practice are triamcinolone, methylprednisolone and betamethasone. The main difference is their duration of action. Some steroids may be preferred by some clinicians depending on their own characteristics: betamethasone has no reported deleterious side-effects, triamcinolone has been shown in some study to have some chondroprotective action and may promote cartilage health. On the other hand, methylprednisolone has been shown to have deleterious effects on cartilage in some studies and therefore is preferred by most clinicians for use in low motion joints such as the tarsometatarsal joint. There is some scientific evidence that hyaluronic acid (HA) exhibits some chondroprotective effects long-term and therefore combination therapy with triamcinolone or betamethasone may be appropriate.
Following intra-articular medication with steroids, five days of rest are generally recommended. I usually recommend two days of stall rest and hand walking followed by three days of light work (lungeing, hacking, light turnout). It has been suggested in the literature that a period of restricted joint motion may reduce clearance of the medication and enable better penetration7.
Finally, infection and laminitis are historically common reported complications from steroids medication and should be discussed with the owner. However, good evidence linking laminitis to corticosteroid injection is lacking and current clinical evidence argues against generalisations of potential risk8 (Table 2).
| Table 2. Intra-articular corticosteroids | |||||
|---|---|---|---|---|---|
| Intra-articular Corticosteroids | Concentration | Dose | Action | Indication | Indicative detection time (hr) |
| Bethamethasone | Celestone 6mg/ml | 4-14mg/joint | Fast onset of action | Shorter acting | 168 (7d) |
| Methylprednisolone | Depo-Medrol 40mg/ml | 20-80mg/joint | Less likely to cause laminitis? | Harmful to cartilage? Dystrophic soft tissue mineralisation Avoid high motion joint |
336 (14d) for 100mg 672 (28d) for 200mg |
| Triamcinolone | Vetalog 6mg/ml | 3-10mg joint ≤18mg horse |
Chondroprotective High motion joints |
May more likely to cause laminitis? | 168 (7d) |
Bisphosphonates
Tildronate (Tildren) and clodronate (Osphos) belong to the non-nitrogenous bisphosphonates class of drugs (Table 3). They are used for their inhibition of bone resorption mainly through inhibition of osteoclasts. Tiludronate slows down bone remodelling and is believed to help restore balance between bone resorption and bone formation.
| Table 3. Tildronate and clodronate | |||
|---|---|---|---|
| Biphosphonate | |||
| Tildren (Tiludronate) | 1mg/kg IV in 10L fluids | Improved lameness score | Can cause colic symptoms |
| Osphos (Clodronate) | 1.8mg/kg IM | Ease of administration | Can cause colic signs, reaction at injection site Some concerns over renal function |
Bisphosphonates also have some reported anti-inflammatory properties by decreasing the amount of nitric oxide and cytokines released from activated macrophages. They also inhibit the secretion of cartilage-degrading enzymes induced by IL-1 in the chondrocyte or the synovial cell9,10.
There are several scientific studies demonstrating the use of bisphosphonates in the horse for applications such as subchondral bone maladaptation and fatigue, disuse osteoporosis, bone marrow oedema syndrome, OA, in tendon and ligament healing (particularly at sites of entheses where bone lysis has occurred), navicular disease and thoracolumbar spine osteoarthritis for example11-13. The age of horses being administered bisphosphonates has been an area of increasing interest due to perceived increased fracture risk in young horses. There are also some reported undesirable effects associated with the use of these products, in particular a significant increase in creatinine was noted after clodronate administration (1.8mg/kg), however values remained within normal limits. Additionally colic signs have been described with the use of bisphosphonates14.
Sodium hyaluronate
Hyaluronate (HA) is mainly responsible for the visco-elasticity and boundary lubrication properties within the joints. Exogenous hyaluronate has also been reported to have anti-inflammatory properties via inhibition of macrophage and granulocyte chemotaxis, inhibition of lymphocyte migration, and reduction of granulocyte and macrophage phagocytosis14,15. Hyaluronic acid is most commonly used for the treatment of joint diseases and tendon lesions. It has the advantages that it can be administered orally, intravenously and intra-articular/intrathecal. Some in vivo studies have shown its ability to reduce joint effusion and degree of lameness together with a certain protective effect on cartilage16,17.
Similarly, intra-thecal injections of HA improved tendon healing and reduced the number of adhesions formed between the tendon and sheath in some studies. It has been proposed that the molecular weight of the product is an important consideration, however there is no consensus to date as to the best formulation for clinical outcomes. One study found that found that horses treated with HA of a molecular weight greater than 2 × 103 kDa were sound significantly longer compared to other formulations.
IV administration is recommended once or twice (Hyonate) or once weekly for a total of three treatments (Legend) (Table 4). In addition, HA can be administered intraarticularly/intrathecal with a combination of other treatment such as steroids. Time of administration has also been considered but to date there is no clear evidence as to the best course of action18,19.
| Table 4. Sodium hyaluronate | |||
|---|---|---|---|
| Trade name | Concentration | Presentation | Recommendations |
| Hyalovet | 10mg/ml | 2ml syringe | 2ml in small/medium joints, no more than twice weekly, no to exceed 4 weeks |
| Hyvisc | 11mg/ml | 5ml syringe/2ml dose | 2ml in small/medium joints4ml larger joints Once weekly for 3 treatments |
| Hylartin V | 10mg/ml | 2ml syringe | 2ml in small/medium size joint 4ml large joints Once weekly for 3 treatments |
| Legend | 10mg/ml | 2ml vial | 2ml once weekly for 3 treatments |
2.5% polyacrylamide hydrogel
Recently, some other products have come on the market. One of the most widely used in the UK is the 2.5% intra-articular polyacrylamide hydrogel or Arthramid. Polyacrylamide gel acts differently from the products already described as it is integrated to the synovial membrane. As such, it does not have effects on pro-inflammatory cytokines. There are a few proposed mechanisms of action for these products, the main one being its effect on the synovial membrane which may reduce pain associated with joint capsule stiffness.
Being a highly viscous product, it may also provide protection to the articular cartilage through its viscoelastic properties20.
From the available literature, these products are best indicated for use in the early stages of OA. Most horses will respond within one week of treatment, but full response may be delayed up to a few weeks to a few months. In most cases where the diagnosis is complete and accurate, one injection only is needed but further injections may be considered 6 to 24 months following the first injection pending on clinical response.
The horse should not have received prior intra-articular medication within two months prior to treatment. It is also reported that the gel is best administered alone. Based on its mechanism of action, this product is not listed as a doping product to date which makes it advantageous in addition to not having the laminitis associated risks of corticosteroid use21-25 (Table 5).
| Table 5. Other IA | |||
|---|---|---|---|
| Other IA | Dose | Indications | Limitations |
| Hyaluronic acid | 11mg/joint-22mg/joint | Chondroprotective effects | Post injection reaction/flare |
| Platelet rich plasma | 2ml-6ml/joint | Available in field | Variable response Can cause reaction |
| Mesenchymal stem cells | 10-20 million/horse | Regenerative properties Some positive results on horses with soft tissue injury |
No significant effect on naturally occurring OA Cost Postinjection reactions Do not use with antibioticss |
| Polyacramide gel | 1ml-3ml into affected joint | Long lasting Reduced laminitis risks |
Cost |
Biologics
Biologics and regenerative medicine therapeutics are growing in equine performance practice. Several products are now available, which can be divided into minimally processed orthobiologics obtained from blood such as autologous conditioned serum and platelet-rich plasma and products obtained after tissue is harvested and processed such as bone marrow aspirate26,27.
Autologous conditioned serum
Autologous conditioned serum (ACS) is harvested after exposition of blood to activating surfaces, resulting in production of several anti-inflammatory cytokines and growth factors by mononuclear cells and degranulated platelets. It is a cell-free product obtained from the liquid phase of blood after coagulation (coagulation takes place during a defined conditioning/incubation phase) and centrifugation28,29.
While ACS contains globulin and albumin it is free of coagulation factors such as prothrombin. Injection protocols may be highly variable depending on the clinical presentation and veterinarian’s preference, but a series of injections one week apart for three to five treatments is commonly performed; again joint and tendon injuries are the most common indications30,31.
Platelet rich plasma
Platelet rich plasma (PRP) is a processed orthobiologic obtained from the liquid phase of blood through centrifugation to increase platelet concentration compared with whole blood. When compared to ACS, it is obtained from anticoagulated blood without incubation.
Platelet rich plasma has been classified in different categories depending on its leukocyte count and activation status: pure PRP which has low WBC and is not activated, non activated leukocyte-rich PRP, pure platelet-rich fibrin which has low WBC and is activated, and activated leukocyte-rich platelet-rich fibrin32,33.
The most common indication for PRP therapy are tendon and ligament lesions followed by joint therapy. Some recent studies have shown improved clinical outcomes and reduced post-injection pain when leukocyte-reduced PRP is used and a longer duration of benefit following a delay of clinical onset (Table 6).
| Table 6. Polyacrylamide hydrogel (PAAG) | |
|---|---|
| Joint | Recommended dose of 2.5% PAAG (ml) |
| Distal interphalangeal (coffin joint) | 2 |
| Proximal interphalangeal (pastern joint) | 1 |
| Fetlock joint | 2 |
| Middle carpal | 2 |
| Tarsometatarsal/distal intertarsal | 1 |
| Talocrural | 2-3 |
| Femoropatellar | 2-3 |
| Lateral/medial Femorotibial |
2-3 |
| Temporomandibular | 1 |
Additionaly, the leucocyte-to-platelet ratio is important in clinical cases and it seems that leuko-reduced PRP is preferred for used in joints since this is supported by the highest level of evidence in human for treatment of mild-to-moderate arthritis34.
Bone marrow aspirate
Aspiration of bone marrow is usually performed from the sternum in horses. Other sites include the tuber coxae in horses three years of age or less and the wing of the ilium. This provides components desired for tissue regeneration, including a scaffold, cells, and bioactive signa.
Among those, mesenchymal stem cells (MSC) can be found in low quantity in bone marrow concentrate (BMC); recently it was shown, however, that both MSC secretome and BMC can recruit MSCs. Although further studies are needed, there is good evidence in the literature that autologous MSCs improve tendon repair in the horse. At this time, the mode of action is still uncertain but differentiation into tendon cells and regeneration of tendon tissue and modulation of inflammation and resulting repair process or both are the proposed mechanism to date.
Similarly, further studies are needed on the use of MSC in joint treatment but in vitro studies have demonstrated that MSCs produce cartilage-specific substances that could improve cartilage repair, such as glycosaminoglycans, and collagen type II.
Further studies have shown improvements in acute cartilage injury and intra-articular fracture. The main clinical application of BMC should be considered for enhanced cartilage defect repair, alleviation of osteoarthritis symptoms, bone regeneration particularly in cystic lesions, and suspensory desmitis.
In one study, intralesional injection of BMC into the suspensory ligament enhanced functional tissue regeneration without evidence of soft tissue mineralisation35-37.
In addition, mesenchymal stem cells (MSCs) modulate the immune system. Therefore, they could be exciting potential treatment of inflammatory conditions in the horse.
As mentioned before, further research is needed to determine optimal cell type/source, preparation, route of delivery, dose and schedule for treating horses.
Conclusion
While NSAIDs and corticosteroids remain the most common treatment used in equine performance to date, there are other products available.
 There is also considerable research being done on developing new products that will hopefully be available on the market in the next few years.
There is also considerable research being done on developing new products that will hopefully be available on the market in the next few years.
Among them, there seems to be some promise in the use of synovium secretome as a disease-modifying treatment for equine osteoarthritis.
References
- May SA and Lees P (1996). Nonsteroidal antiinflammatory drugs. In McIlwraith CW and Trotter GW (eds), Joint Disease in the Horse, WB Saunders, Philadelphia: 223–237.
- Kallings P (1993). Nonsteroidal anti inflammatory drugs, Vet Clin North Am Equine Pract 9(3): 523-541.
- Lees P and Higgins AJ (1985). Clinical pharmacology and therapeutic uses of non-steroidal anti-inflammatory drugs in the horse, Equine Vet J 17(2): 83-96.
- Doucet MY, Bertone AL, Hendrickson D et al (2008). Comparison of efficacy and safety of paste formulations of firocoxib and phenylbutazone in horses with naturally occurring osteoarthritis, J Am Vet Med Assoc 232(1): 91-97.
- De Grauw JC, van de Lest CHA, Brama PAJ et al (2009). In vivo effects of meloxicam on inflammatory mediators, MMP activity and cartilage biomarkers in equine joints with acute synovitis, Equine Vet J 41(7): 693-9.
- Harkins JD, Carney JM and Tobin T (1993). Clinical use and characteristics of the corticosteroids, Vet Clin North Am Equine Pract 9(3): 543-562.
- McIlwraith CW (2010). The use of intraarticular corticosteroids in the horse: what is known on a scientific basis? Equine Vet J 42(6): 563-71.
- Bathe, AP (2007). The corticosteroid laminitis story: 3. The clinician's viewpoint, Eq Vet J 39(1): 12-3.
- Yocom A, Contino E and Kawcak C (2023). Review of the mechanism of action and use of bisphosphonates in horses, J Equine Vet Sci 127: 104503.
- Soto SA and Barbará AC (2014). Bisphosphonates: pharmacology and clinical approach to their use in equine osteoarticular diseases, J Equine Vet Sci 34(6): 727-737.
- Denoix JM, Thibaud D and Riccio B (2003). Tiludronate as a new therapeutic agent in the treatment of navicular disease: a double-blind placebo controlled clinical trial, Equine Vet J 35(4): 407-413.
- Coudry V, Thibaud D, Riccio B et al (2007). Efficacy of tiludronate in the treatment of horses with signs of pain associated with osteoarthritic lesions of the thoracolumbar vertebral column, Am J Vet Res 68(3): 329-337.
- Gough MR, Thibaud D and Smith RK (2010). Tiludronate infusion in the treatment of bone spavin: a double blind placebo controlled trial, Equine Vet J 42(5): 381-387.
- Ehrle A, Fürst A and Lischer C (2013). Efficacy and adverse effects of joint medication in the horse. A review of the literature. Part 1: Conventional intra-articular drug therapy and risks of joint injection in horses, Pferdeheilkunde Eq Med 29(1): 54-64.
- Santangelo KS, Johnson AL, Ruppert AS et al (2007). Effects of hyaluronan treatment on lipopolysaccharide-challenged fibroblast-like synovial cells, Arthritis Res Ther 9(1): 204.
- Ferris DJ, Frisbie DD, McIlwraith CW et al (2011). Current joint therapy usage in equine practice: a survey of veterinarians 2009, Equine Vet J 43(5): 530-535.
- Williams VS (2007). Intraarticular hyaluronic acid supplementation in the horse: the role of molecular weight, J Equine Vet Sci 27(7): 298-303.
- Spurlock GH, Spurlock SL and Parker GA (1989). Evaluation of hylartin-V therapy for induced tendonitis in the horse, J Equine Vet Sci 9(5): 242–246.
- Gaughan EM, Nixon AJ, Krook LP, et al (1991). Effects of sodium hyaluronate on tendon healing and adhesion formation in horses, Am J Vet Res 52(5): 764-773.
- Tnibar A (2022). Intra-articular 2.5 per cent polyacrylamide hydrogel, a new concept in the medication of equine osteoarthritis: a review, J Equine Vet Sci 119: 104143.
- de Clifford LT, Lowe JN, McKellar CD, Bolwell C and David F (2019). Use of 2.5 per cent cross-linked polyacrylamide hydrogel in the treatment of joint lameness in a population of flat racing Thoroughbreds: a pilot study, J Equine Vet Sci 77: 57-62.
- Tnibar A, Schougaard H, Camitz L et al (2015). An international multi-centre prospective study on the efficacy of an intra-articular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up, Acta Vet Scand 57(1): 20.
- AP Bathe, RM Read and C Briggs (2016). Intra-articular polyacrylamide hydrogel for the treatment of 20 horses with non-responsive osteoarthritis of the interphalangeal joints: a prospective study, Veterinary Orthopaedic Society, 43rd Annual Conference Abstracts, https://bit.ly/42Vtq7G
- Azambujada Silva Xavier A, Pinto da Rosa P, Brum Mackmill L and Buttow Roll VF (2021). An assessment of the effectiveness of hyaluronic acid and polyacrylamide hydrogel in horses with osteoarthritis: systematic review and network meta-analysis, Res Vet Sci 134: 42-50.
- Travis de Clifford L, Lowe JN, McKellar CD, McGowan C and David F (2021). A double-blinded positive control study comparing the relative efficacy of 2.5 per cent polyacrylamide hydrogel (PAAG) against triamcinolone acetonide (TA) and sodium hyaluronate (HA) in the management of middle carpal joint lameness in racing Thoroughbreds, J Equine Vet Sci 107: 103780.
- Ehrle A, Fürst A and Lischer C (2013). Efficacy and adverse effects of joint medication in the horse. A review of the literature. Part 2: Regenerative and innovative joint medication in the horse, Pferdeheilkunde Eq Med 29(2): 212-218.
- Schnabel LV (2023). Considerations for the use of biologic and regenerative therapies in equine practice, Vet Clin North Am Equine Pract 39(3): xiii-xiv.
- Ortved KF (2023). Equine Autologous Conditioned Serum and Autologous Protein Solution, Vet Clin North Am Equine Pract 39(3): 443-451.
- Tokawa PKA, Brossi PM, Baccarin RYA (2022). Autologous conditioned serum in equine and human orthopedic therapy: a systematic review, Res Vet Sci 146: 34-52.
- Geburek F, Lietzau M, Beineke A, Rohn K and Stadler PM (2015). Effect of a single injection of autologous conditioned serum (ACS) on tendon healing in equine naturally occurring tendinopathies, Stem Cell Res Ther 6(1): 126.
- Camargo Garbin L, Morris MJ (2021). A Comparative Review of Autologous Conditioned Serum and Autologous Protein Solution for Treatment of Osteoarthritis in Horses, Front Vet Sci 19(8): 602978.
- Camargo Garbin L, Lopez C and Carmona JU (2021). A Critical Overview of the Use of Platelet-Rich Plasma in Equine Medicine Over the Last Decade, Front Vet Sci 31(8): 641818.
- Geburek F, Gaus M, van Schie HT, Rohn K and Stadler PM (2016). Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies – a randomized prospective controlled clinical trial, BMC Vet Res 12(1): 191.
- Moraes AP, Moreira JJ, Brossi PM, Machado TS, Michelacci YM and Baccarin RY (2015). Short- and long-term effects of platelet-rich plasma upon healthy equine joints: clinical and laboratory aspects, Can Vet J 56(8): 831-838.
- Schnabel LV, Mohammed HO, Jacobson MS et al (2008). Effects of platelet rich plasma and acellular bone marrow on gene expression patterns and DNA content of equine suspensory ligament explant cultures, Equine Vet J 40(3): 260-265.
- Smith JJ, Ross MW and Smith RKW (2006). Anabolic effects of acellular bone marrow, platelet rich plasma, and serum on equine suspensory ligament fibroblasts in vitro, Vet Comp Orthop Traumatol 19(1): 43-47.
- Fortier LA (2023). Equine bone marrow aspirate concentrate, Vet Clin North Am: Eq Pract 39(3): 453-459.
