22 Mar 2022
Strangles: an old acquaintance that’s still not fully understood
Leticia Macias Bricio describes clinical signs, diagnosis and disease prevention, as well as outbreak management and surveillance efforts.

How to palpate the retropharyngeal lymph nodes.
Strangles is an equine infectious disease reported for the first time in 1251, but historians are almost certain the illness existed before then.
It presents as a lymphadenopathy generally associated with fever, caused by the bacteria Streptococcus equi subspecies equi (S equi), and commonly affecting head and neck lymph nodes, which become enlarged and painful until they mature into abscesses. The size of these can sometimes cause difficulty eating (dysphagia) and breathing – because of this, the name of “strangles” was coined.
Nowadays, it is still one of the most spread equine infections worldwide, causing important welfare and financial damage in the horse industry. It is a reportable disease in the US, but not in the UK, where 600 new outbreaks are estimated to occur every year (Waller, 2014) – and the truth is that it can affect horses of any age, sex, breed and condition, no matter the location or the type of facilities.
While trying to learn the pathogenic mechanisms to find an effective vaccine against the bacteria, the goal of eradicating strangles relies on good management of outbreaks and prevention of new ones, mainly by targeting and treating asymptomatic carriers, which are difficult to identify and responsible for a good number of reinfections.
Until this moment, no changes on the genome, methylome or transcriptome have been observed between bacteria causing clinical signs and those isolated from silent carriers, which indicates that perhaps an adaptive response occurs from the host (Morris et al, 2021) and would open new lines of investigation to understand how the silent carrier status is achieved.
The bacteria
All Streptococci share some general characteristics (Slater, 2007):
- Gram-positive cocci approximately 1μm in diameter
- occur as pairs or chains depending on the culture environment: usually as pairs (diplococci) in the animal, but as elongated chains in vitro; diplococci are more virulent
- facultative anaerobes
- catalase and oxidase negative
- non-motile and non-spore-forming (therefore, limited environmental persistence and reservoir of infection)
- β-haemolysis (complete) on blood agar plates (except Streptococcus pneumoniae, which is α-haemolytic) thanks to cytolytic toxins such as streptolysin or streptokinase.
- Lancefield group C (except S pneumoniae, which has no Lancefield group antigen)
- opportunist pathogens of the respiratory tract
- cause both mucosal respiratory infections and invasive systemic disease
- host range varies from strictly equids (S equi) to a variety of animal species and humans (S zooepidemicus, S pneumoniae)
- variety of virulence and defence mechanisms, including the antiphagocytic capsule and surface (M) protein
- variably transient immunity following natural infection; some species have huge antigenic diversity between strains with little cross-protection
It is important to remember that, opposite to other Streptococci, S equi is not considered a normal commensal in the equine airways and is always pathogenic (Barquero et al, 2007; Mallicote, 2015).
Gram-positive bacteria are surrounded by a thick peptidoglycan cell wall, but, compared to Gram-negative bacteria, do not have an outer membrane, which makes them weaker in the environment and against antibiotics.
Streptococcus cell walls consist of peptidoglycans and teichoic acid. Many surface proteins are anchored to the cell wall, projecting into the environment. Some of them are known to be involved in specific mechanisms of pathogenesis, such as adhesion to the respiratory epithelium, evasion of phagocytosis and immunogenicity (Slater, 2007; antiphagocytic SeM protein, H factor binding Se18.9, Mac protein and other undetermined antiphagocytic factors released by the organism; Boyle et al, 2018).
The cell wall is surrounded by a loose network of polysaccharides known as the “cell capsule”, which, in Streptococcus species, consists of hyaluronic acid, except for S pneumoniae. Hyaluronic acid is non-immunogenic, protects the bacterium against the horse’s inflammatory defences and contributes to evade phagocytosis.
The scientific community has concluded that S equi has evolved from S zooepidemicus, with which it shares more than 97% of its DNA. This could be why horses that have previously suffered from S zooepidemicus infections display milder signs of strangles when infected with S equi (Waller, 2014); however, this can make diagnosis of strangles more challenging.
S equi is prevalent worldwide, very homogeneous and an apparently clonal microorganism. For many years, it was believed a single serotype existed, but it has been demonstrated that at least seven different subtypes exist, with particular geographical distribution, and new strains are still being discovered (Tartor et al, 2020; Dong et al, 2019). On the top of this, bacterial populations in a same region can change over time, making the goal of effective universal, long-lasting vaccines more complicated.
Pathogenesis
The bacteria enter the oronasal passage through direct contact with infected horses (nose to nose) or with fomites such as drinking troughs, feeding buckets, brushes, head collars, bridles, and staff and veterinarians’ hands and clothes.
It reaches the pharyngeal tonsillar tissue, and within three hours progresses to the subepithelial cells and the pharyngeal lymph nodes, so it is no longer evident on epithelial tissue. Because of this, nasopharyngeal swabs may not detect the bacteria 24 hours post‑infection (Newton, 2011a).
Neutrophils fail to prevent bacterial multiplication due to S equi’s ability to evade phagocytosis. This will result in the typical lymphadenopathy with abscess forming after three to four days.
Fever occurs 3 to 14 days after exposure, generally before lymph node abscess maturation and before the onset of bacterial shedding, so it is a good indicator for isolating the suspicious horse before infecting other animals (Newton, 2011a). This is followed in two to three days by nasal shedding, which persists normally for two to three weeks, although in some horses clearance can be delayed by up to four to six weeks, especially if guttural pouches are affected.
An anatomical overview of the upper aspect of an equine left guttural pouch and the main structures found in it. (Click each hotspot for details)
When the abscesses rupture, purulent material is released into the horse’s environment and airways, spreading the disease and infecting other individuals.
Bacteraemia has been observed 6 to 12 days after experimental infection with a virulent strain (Cordoni et al, 2015), which explains the uncommon spread to distant lymph nodes (“bastard strangles”).
Strangles mortality is usually low (Barquero et al, 2007; Boyle et al, 2018; Slater, 2007), but its high morbidity – plus the short-term and long-term consequences from the outbreaks – reinforce the importance of keeping this disease under control.
Records show that 10% of the affected horses become long-term shedders (carriers), and many investigations are trying to find the reason why patients remain asymptomatic for months or even years before suddenly shedding again to infect naive individuals, which will suffer from the typical symptoms.
Opposite to S zooepidemicus, S equi is quite host‑specific and will affect only Equidae, but some rare cases of infection in immunodepressed people have been observed. This, combined with the fact we can still find S zooepidemicus as a concomitant bacterium, make hygiene and control measures even more important to prevent zoonosis.
An anatomical overview of the equine pharynx. (Click each hotspot for details)
Clinical signs
Due to bacterial proliferation and the failure of the first immune response, pyrexia and lymphadenopathy will appear 3 to 14 days after exposure.
Generally, submandibular and retropharyngeal lymph nodes are affected, but any node in the head or neck is susceptible. If this is the case for thoracic lymph nodes, serious complications can occur from tracheal air flow restriction.


Periorbital swelling has also been observed in some cases.
Depression and inappetence are typical due to the fever, pharyngitis and/or dysphagia caused by the swollen retropharyngeal lymph nodes, as well as an occasional cough. If the swelling is very severe, breathing can be compromised and an emergency temporary tracheostomy may be required.
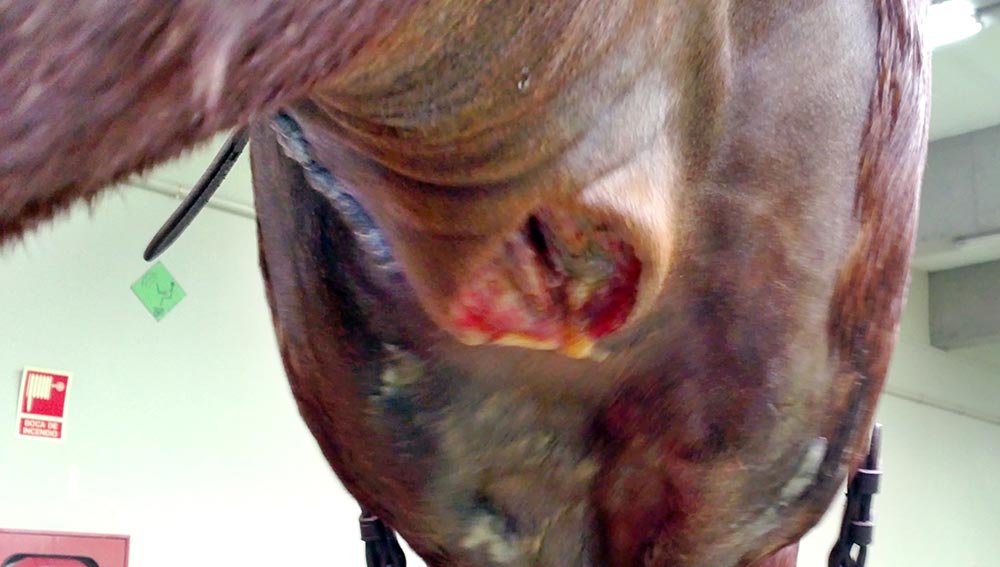
Between seven days and four weeks after infection, mature abscesses will break and drain into the airways or the guttural pouches (GP), or out into the environment. The first two options will result in the typical purulent nasal discharge.
Field and experimental data support that disease severity is correlated with the dose and frequency of the infectious challenge (Newton, 2011a).
Additionally to chronic carriage in the GPs, other possible complications of S equi infection exist.
Metastatic abscessation is better known as “bastard strangles”, and can be observed when the bacteria travels via lymphatic or blood vessels to different locations in the body and form abscesses. Thoracic and mediastinal abscesses could lead to respiratory distress and pneumonia. Depending on where the abscesses are formed within the abdominal cavity, signs such as colic or weight loss can be observed, with or without respiratory alterations. Disease processes such as septic arthritis, meningitis and myocarditis have also been noted in case literature.
Immunological complications are unusual. The most common one is purpura haemorrhagica (PH; Cordoni et al, 2015), a type‑three immune‑mediated hypersensitivity reaction that leads to vasculitis due to deposition of immune complex within the vascular intima.
Signs are characterised by ventral oedema, including distal limbs, possibly associated with skin necrosis, petechial haemorrhages and ecchymosis. It must be understood that vasculitis will not only affect the skin, but also internal organs. Normally it appears three to four weeks after strangles infection or following vaccination.
Horses that have been previously hypersensitised are at higher risk of developing PH and it has been suggested that increased IgA titres are related to PH severity, while IgG antibodies may have a protective role. Diagnosis can be confirmed by serology, where SeM antibody titres will be 12,300 or greater, and by biopsy of the affected skin.
Myositis is a rare consequence of PH, but it can have different presentations and a high fatality rate (Cordoni et al, 2015). Diagnosis will be confirmed, again, by biopsy and serology.
Most horses will recover from uncomplicated strangles in a period of weeks. After two weeks, an adapted immune response is established that will develop an effective protection against S equi in 75% of patients, lasting about five years (Cordoni et al, 2015, Newton, 2011b).
About 10% of affected horses will remain as carriers due to empyema not being cleared completely from the GPs or sinuses. This pus dries and forms chondroids that can persist in the animal’s body for years, with the horse becoming an intermittent shedder.
Carriers, older horses with residual immunity and foals with waning maternal antibody protection can remain completely asymptomatic or develop a mild modality of the disease known as atypical strangles. Symptoms will not be so obvious – without swelling of the lymph nodes, nasal discharge and even without fever – but they will still shed the bacteria and infect other horses, and because of it, targeting them is key in eradicating strangles.
Diagnosis
When the first clinical signs are noticed, a general haematology and biochemistry will only show the presence of an active inflammatory process.
Bacterial culture has been the gold standard method to identify the pathogen, but due to metabolic similarities with other bacterium from the same family (S zooepidermicus), plus the fact S equi growth can be inhibited in the presence of other species, getting an accurate diagnosis can be difficult.
Classically, samples from draining abscesses have been submitted for culture, as well as nasal swabs, nasopharyngeal swabs or samples from both GPs.

Due to bacterial sensitivity during transport and to sunlight, samples should arrive at the lab in good condition to be processed successfully.
S equi must compete with other β‑haemolytic bacteria that may be present in the equine airways as a commensal. Also, the bacteria may not be found in the respiratory epithelium if the swabs have been taken at a very early stage. All this can lead to false negatives on this test (Boyle et al, 2018).
Blood ELISA serology merely allows the clinician to determine exposure and it could lead to a false negative result if the sample was taken too early. So, ideally, it should be compared with a paired sample two to three weeks after the first one, and should be followed by culture and PCR.
Strangles ELISA tests have been typically orientated to detect antibodies for the surface protein SeM, but the inability to differentiate vaccination from infection – and some cross‑reaction with an analogous protein in S zooepidemicus – has led to the development of a new dual iELISA test with a specificity of 99.3%. This is based on detecting antibodies against the N-terminal portion of SeM, which is unique to S equi, and antibodies against the gen SEQ2190 (antigens A and C, Newton, 2011a; Slater, 2007).
Serology is not able to differentiate infected animals from those that have been recently in contact with the bacteria, and because the antibody titres will remain high for several weeks, it is not considered a definitive diagnostic test.
More recently, a study on a charity welfare centre in the UK has shown serology antigens A and C detection could still lack significant sensitivity when identifying carriers, as an important number of asymptomatic animals, positive to GP PCR, had false negative results on the serology test (Durham and Kemp‑Symonds, 2021). This data highlights the uncertainty of relying on a single blood sample when moving horses to a new yard.
Since the PCR test was established for S equi, great advances on specificity and rapidity of diagnosis have been made. Some problems have also arisen with PCR specificity and sensitivity, due to similarities with other β-haemolytic bacteria and variability on the SeM protein 5’ region.
The latest test is a triplex quantitative PCR (qPCR) that analyses two S equi specific genes: eqbE and SEQ2190, plus an internal control strain for S zooepidemicus (Newton 2011a, Slater, 2007), and it has become the gold standard diagnostic method to detect strangles.
To get quicker results, and to start the isolation and security protocols earlier when suspecting strangles, some investigation lines are focused on developing faster PCR assays or “patient-side” devices (point of care PCR) to allow the clinician to perform the test at the yard. This is very promising, but it still has not achieved enough reliability-cost balance to be performed in the field (Willis et al, 2021).
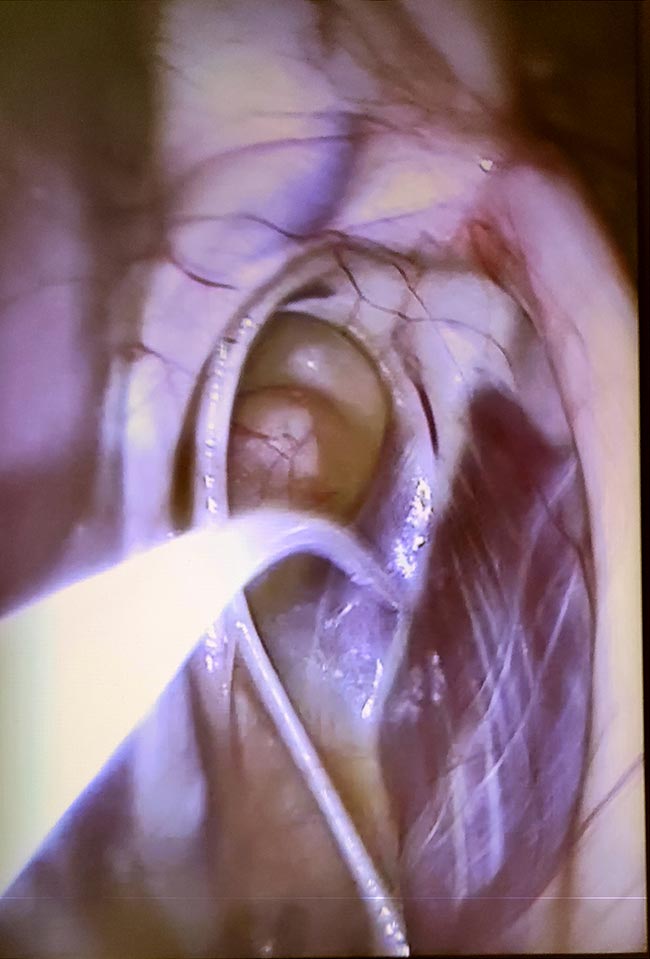
Several studies have compared culture and PCR for S equi from different samples. Optimal sampling would be a qPCR on a needle aspirate from swollen/draining lymph nodes. When this cannot be performed (such as immature abscesses or complicated anatomical region to access), studies point at qPCR – ideally combined with culture – from bilateral GP lavage (GPL) as the most specific and accurate test, which is able to detect not only convalescent horses, but also asymptomatic carriers (Barquero et al, 2007; Boyle et al, 2016; 2018).
The Horserace Betting Levy Board (HBLB) Code of Practice for Strangles in the UK proposes the following techniques for diagnosis of an active infection: a qPCR and culture, from three nasopharyngeal swabs taken one week apart, or from a GPL where the swabs cannot reach.
To identify carriers, they recommend performing a unique GPL and nasopharyngeal swab at the same time, rather than the three swabs. They highly recommend performing a qPCR together with culture, or just a qPCR rather than culture, because of its higher specificity and sensitivity.
Other techniques – such as nasopharyngeal and nasal washes – have been tested, but GPL using qPCR of the obtained samples still remains the best technique to identify infected horses and carriers (Boyle et al, 2016; 2017). Plus, it allows visual examination of the upper airways and GPs to spot abnormalities. Because of this, the HBLB states this is the preferred method.
Prevention
The best way to avoid outbreaks is avoiding dissemination – firstly by quarantining new horses arriving on the yard for three weeks, then by carrying out a paired blood ELISA serology.
As a minimum, it is recommended to perform two paired serology tests 15 days apart. In practice, this advice is seldom followed, as many yards admitting a new horse usually only require one negative blood test.
In the case of a positive or borderline serology result, without any clinical signs, further investigation via GPL should be carried out. If this is positive, treatment of the GP in situ with penicillin is advocated and repetition of the GPL two to three weeks later is required (Mallicote, 2015; Waller, 2014).
If clinical signs develop, the horse must be isolated and investigated, and the premises where it resides should be closed and a quarantine imposed.
Other measures – such as avoiding nose‑to‑nose contact with other horses when going to shows, avoiding sharing buckets, and cleaning hands after dealing with other participants – are also recommended (Sweeney et al, 2005).
Vaccination
Findings on molecular structure and deeper knowledge on S equi genome bring hope for achieving effective, safe vaccines, which allow differentiation between vaccinated and infected animals (DIVA); however, at the moment, vaccination is very controversial (Barquero et al, 2007).
In general, we find vaccines should be administered only to healthy horses, with a minimum age depending on the vaccine. Due to the serious adverse effects, and the risk of reverting to virulence when dealing with attenuated-live vaccines, these animals should not have suffered the disease or have been in contact with infected animals or carriers in the past year, (Barquero et al, 2007; Mallicote, 2015; Waller, 2014).
Before vaccination, a SeM ELISA could be performed to clear up suspicions: animals at risk of developing purpura will display antibody titres 1:3, 200 or greater, and should not be vaccinated (Boyle et al, 2018; Mallicote, 2015).
Managing outbreaks
Quick identification and isolation of infected animals is essential. The yard must be closed to any movement until the final affected horse has been declared clear from disease. All the owners, staff and veterinary surgeons in the yard should be informed, and efforts should be coordinated to clear the outbreak as soon as possible. Neighbouring yards should be informed as well, so the disease is not spread further.
It is advisable to separate the animals into three groups:
- horses with clinical signs
- horses without signs, but that might have been in contact with infected individuals
- horses that have had no contact with any of the other groups
The three groups should ideally be sheltered in different barns or fields, with additional fencing or the best measures available to avoid direct contact between them.
Special attention should be paid to younger and older animals as they are more susceptible, and managing outbreaks in studs could be even more challenging due to the differing ages and immunity status in this population.
Specific tools and equipment must be assigned to each group without mixing them. Keeping in mind the bacteria can live up to one month in water, extra care should be paid to drinking buckets and troughs.
Sanitary barriers – such as foot baths, hand wash, special/disposable clothes and disposable gloves for dealing with each group – must be used, particularly around the group presenting with clinical signs.
Ideally, specific staff should be designated to deal with each group. If this is not possible, sanitary barriers become even more important.
Husbandry should be performed in sequence – the horses in the clear group should be looked after first, then the ones in the suspicious group and finally the infected patients.
An advantage from S equi is that the bacteria is relatively weak in the environment, not lasting more than three days – and even less if exposed to the sunlight – although wooden surfaces need to be dried for longer.
Regular disinfectants – such as quaternary ammonium compounds, hypochlorite (household bleach) and phenolic compounds, among others – will defeat the bacteria, but it must be remembered that they will be effective only if all the organic material is removed first with foaming soap, otherwise the disinfectants will be deactivated and will not work (Boyle et al, 2018).
Fields holding infected horses should be rested to dry for at least three weeks following any outbreak.
Temperature should be monitored in all the horses twice a day to detect new infections early. A horse showing a high temperature should be moved to the infected group before it starts to shed.
A paired serology test should be performed, ideally on all the horses in the yard, as they will help to identify asymptomatic horses and carriers.

Testing the infected group can be performed by three nasopharyngeal swabs separated one week apart or by bilateral GPL. The latter is the recommended diagnostic method. In any case, samples should be submitted for PCR and culture to increase the sensitivity, but qPCR will be preferred.
Treatment is symptomatic – NSAIDs, draining and cleaning abscesses, increasing appetite, keeping the animals well hydrated and fed, monitoring and optimising breathing if compromised, and so on.
Antibiotic therapy in strangles is controversial. Early treatment with systemic antibiotics may delay the abscesses’ maturation and decrease the immune response, making these animals more susceptible to remain as carriers and to suffer the disease in a second outbreak (Boyle et al, 2018; Duran and Goehring, 2021; Mallicote, 2015; Waller, 2014).
Horses with severe signs, compressed airways, immunosuppression or showing complicated forms of strangles should be treated with a prolonged course of antibiotics. Penicillin is the first option due to the bacteria’s susceptibility and the absence of reported resistances, but depending on the case, other antibiotics – such as ceftiofur or trimethoprim/sulfadiazine – might be considered, although consistent failure of treatment with the final one has been reported (Boyle et al, 2018).
Aminoglycosides should always be avoided, as the bacteria has proved to be consistently resistant. An increased resistance to enrofloxacin has also been observed (Boyle et al, 2018).
Horses confirmed as positive should get a good wash of the GPs with sterile saline, removal of any chondroids identified, and it could be useful to administer antibiotics “in situ”. Because of the GPs’ anatomy, any fluid deposited in them will come out quite quickly, so different formulations of penicillin gels are available to achieve an effective time of contact and higher dose applied locally.
A second GP sampling must be performed. If the results are negative, the individual can go into the clear group. Due to the variability on the number of days of convalescence (two to six weeks), in the case of an outbreak it is recommended to start sampling at least four weeks after the last clinical signs to check clearance and identify carriers, to avoid slower individuals to test positive as carriers because of being sampled too early.
The yard can be reopened three weeks after the final animal showed clinical signs or tested positive, if no new cases are identified.
The population that the author’s practice attended between 2016-17 did not show a high prevalence of active infections, but many horses had been exposed to the bacteria at least once in their lives.
The vast majority of the serology tests were performed without symptomatology, as a previous requirement to move yards. Borderline results in serology or positive serology followed by negative guttural pouch lavage (GPL) could indicate that the exposure took place in the past five years (Cordoni et al, 2015; Newton, 2011b).
Two horses came into a new yard without previous serology. One horse developed nasal discharge during the first week without lymphadenopathy or other clinical signs, while the other did not show signs at all. Once the risk was noticed, both were isolated and an outbreak protocol was imposed in the yard. Both horses underwent a serology test at that time, which was positive, so they had a nasopharyngeal swab done, which was negative for both horses.
However, because of the known lack of sensitivity of the nasal swab if it has been taken too early, and the evident signs displayed by one of the horses, it was followed by GPL, which came back positive for the horse with clinical signs and negative for the other one.
Penicillin treatment was administered in situ and GPL was repeated 15 days later, also for the horse with negative results. As it had been isolated together with the positive horse, it was considered at risk due to close contact.
Serologies were performed on those individuals that could have been in touch with these two, all of them with negative results. Temperatures were taken systematically on all the horses in the yard – no horse developed fever or other signs, and the yard was reopened after the second GPL was negative on the affected horses, three weeks after starting the security protocol.
This example highlights the importance of testing and keeping new arrivals in quarantine before introducing them to the herd. It also helps to demonstrate the higher efficacy of washing the GPs for PCR and culture compared to testing the nasopharyngeal swabs, even in a horse with clinical signs.
Strangles diagnostic methods, 2016-17
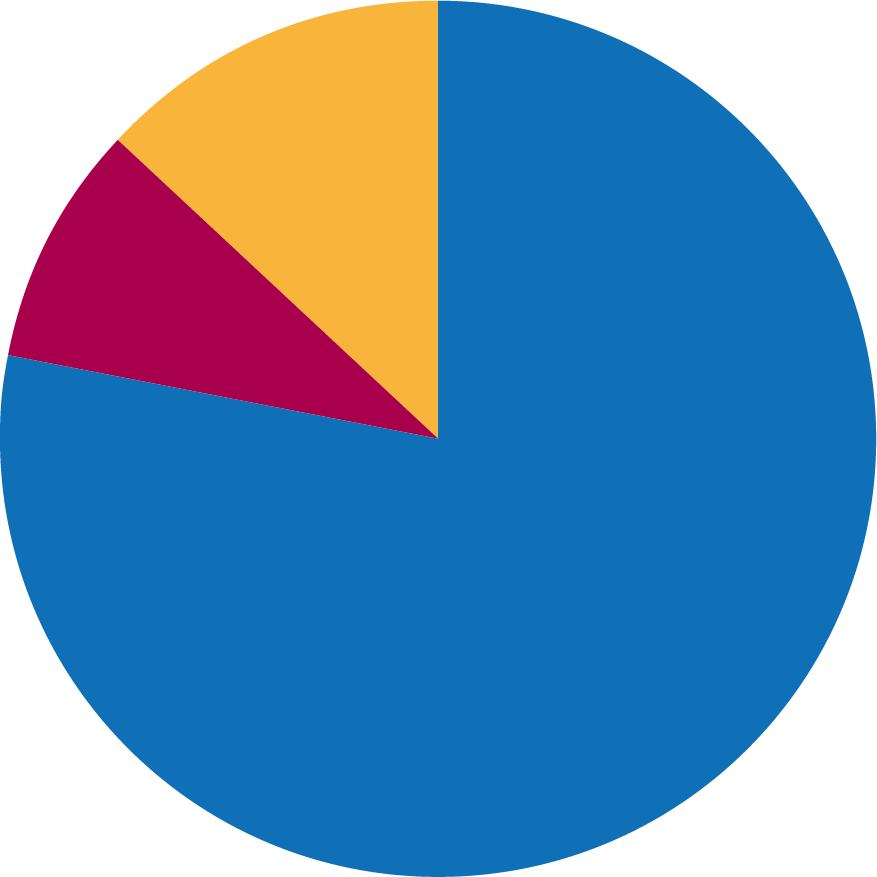
Between 2016‑17, the author’s practice performed 115 serology tests, 13 nasopharyngeal swabs and 19 GPLs.
Serology results, 2016-17
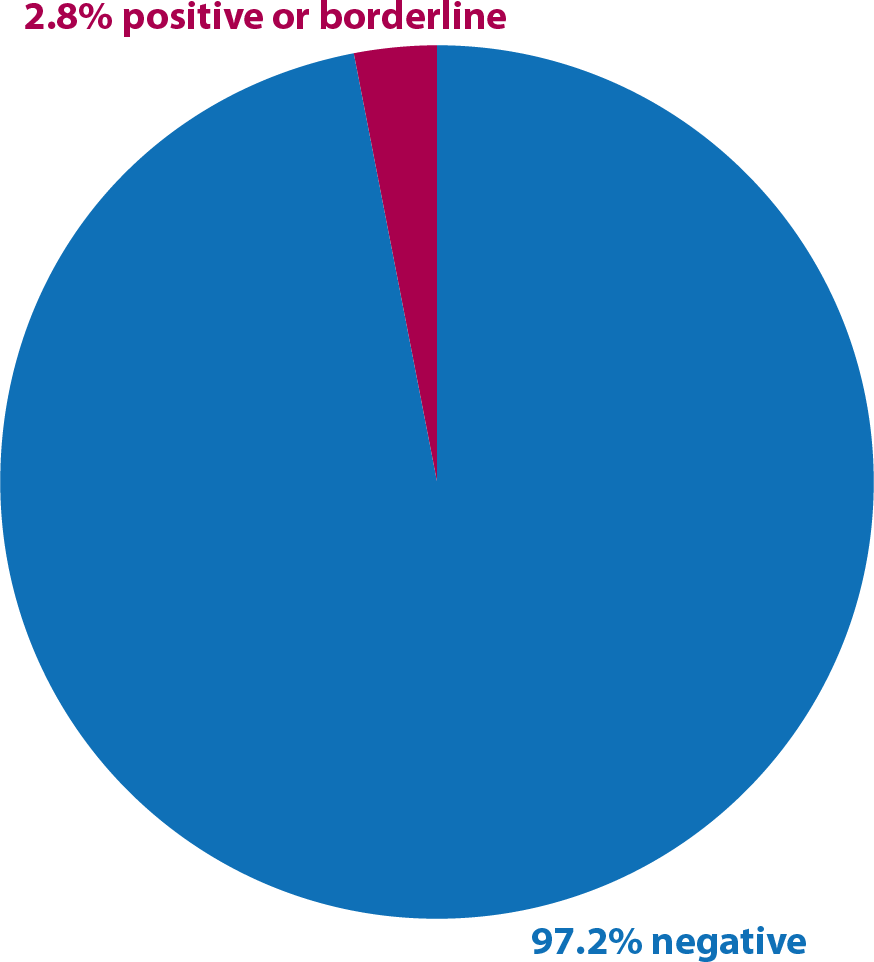
Of the 115 serology tests, most of them were performed as a request to move yards. Only 12 underwent GPL because of showing a positive results or borderline result in paired samples taken two weeks apart.
Results on nasal swabs, 2016-17
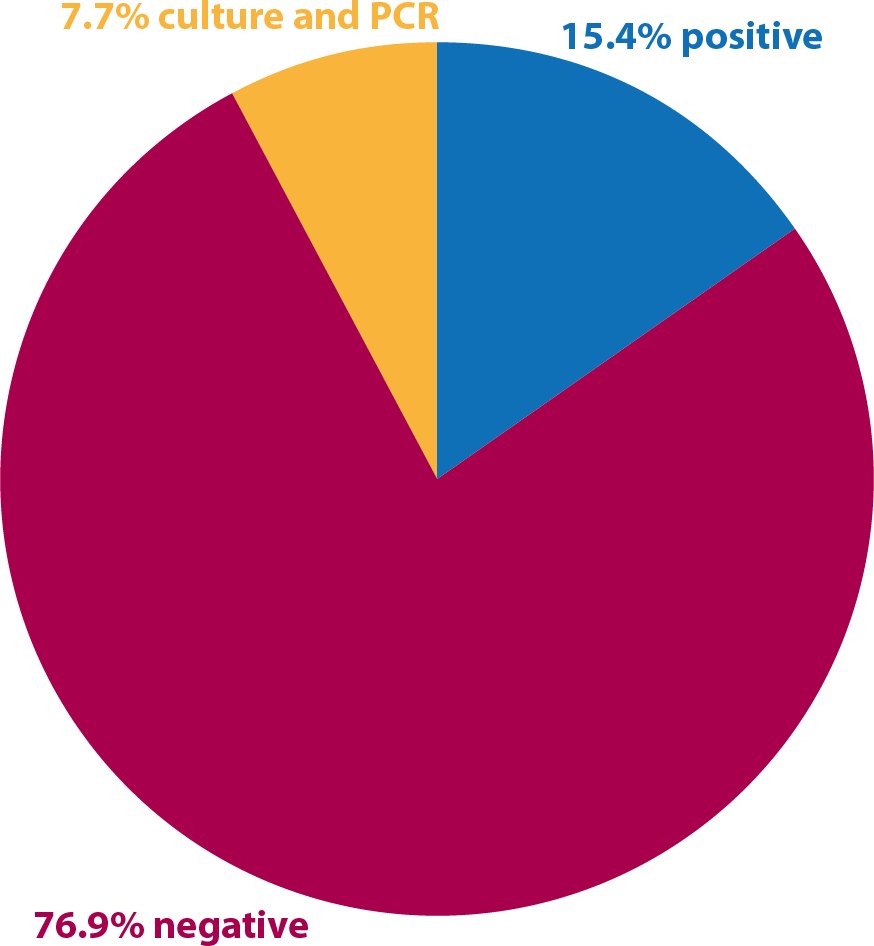
Two cases of nasopharyngeal swabs were positive and underwent GPL. One of these was negative to the PCR, but positive to the culture. Sometimes this can happen due to a polymerase inhibitor secreted by the bacteria when a high amount of microorganisms are present.
GPL, 2016-17
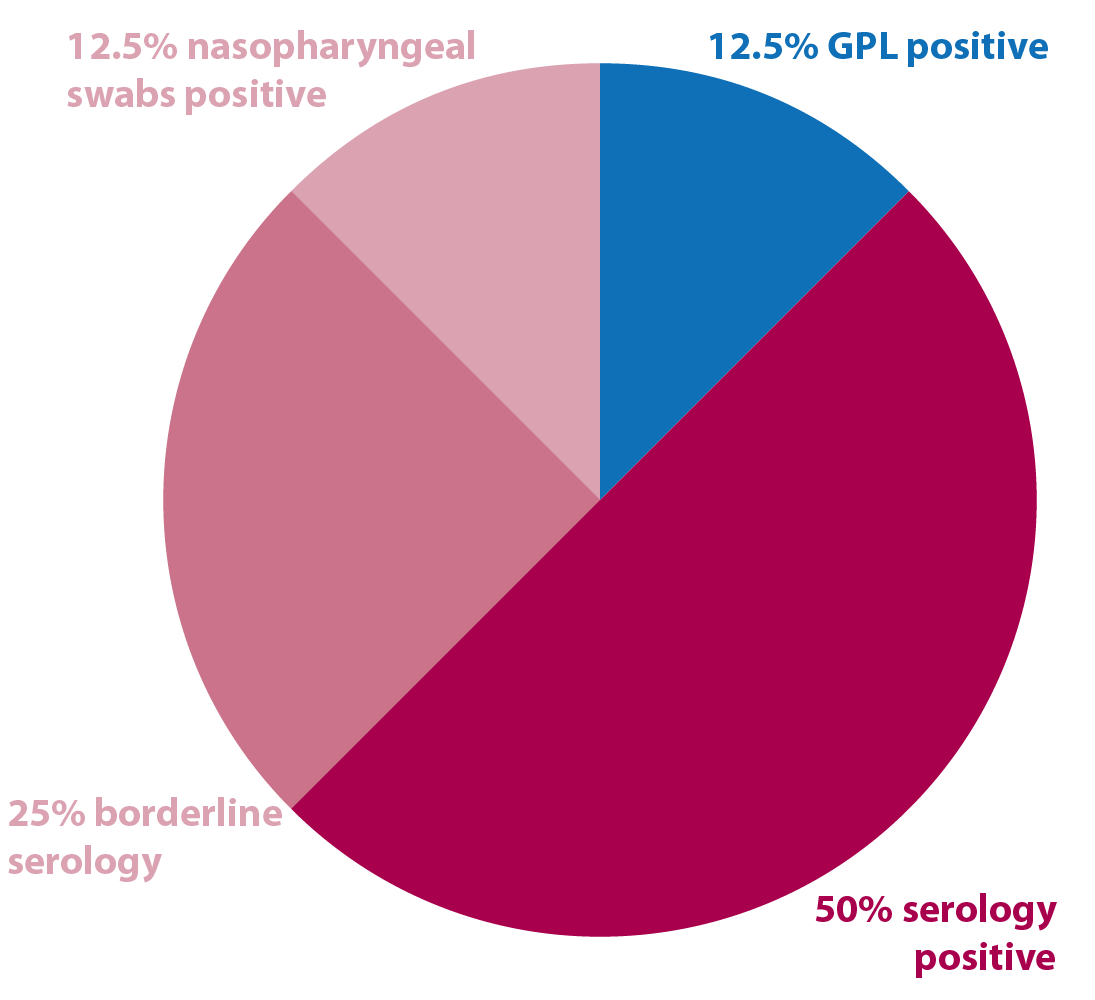
Two cases tested positive. These were put on systemic antibiotics plus one local application of penicillin in both guttural pouches (GPs). Both underwent a second test 10 days later and were negative.
Another case had a repeated GPL, because of living with an infected horse, after the first test was negative.
Between all the negative results on GPL:
- eight cases were performed due to a clear positive serology
- four cases were performed due to two borderline paired results on serology
- two cases were performed due to a positive nasal swab, despite in one of them it not being possible to access GP, but pus coming from the GP was observed on endoscopy
- two cases had negative nasal swab and positive serology, with one case positive on GP qPCR and culture
Conclusions
Information is the first tool to defeat any disease. Owners and equestrians should be familiar with strangles’ key points, how it spreads, clinical signs, and what to do in a potential case or an outbreak.
In the UK, The British Horse Society, BEVA and the HBLB provide plenty of accessible resources on their websites, including advice and directions to prevent outbreaks and how to manage them.
Bringing attention to strangles’ morbidity and wide geographical distribution should encourage owners to report cases and reinforce prevention measures, even when it is not a notifiable disease in the UK, to provide a real picture of its evolution and to take steps towards eradication.
With this goal, a project is under way, sponsored by The Horse Trust, in collaboration with the RVC, and some of the main laboratories and practices in the UK. Surveillance of Equine Strangles is collecting information about reported cases in the UK and analysing the data – classifying it by geographical region, breed, age, sex, type of diagnostic test, type of sampling, time of the year, and more. This data is published online in real time, accessible to everyone.
In recent years, remarkable advances have been made to better understand the pathology, and to provide efficient and quick diagnostic tools. Detecting carriers and treating them is fundamental to preventing new outbreaks from happening, and GPL qPCR has proved to be the gold standard procedure. It is still considered expensive by a lot of clients, and some of them would prefer to start with the three nasopharyngeal swabs instead.
In the author’s experience, the process of carrying out three nasopharyngeal swabs is often more expensive and slower than a GPL. This is because the latter requires only one visit and produces a definitive result sooner, plus the upper airways can be examined and treatment started at the time if necessary.
Knowing the epidemiology and pathology helps to implement barriers for the bacteria before spread occurs. Quarantines and serology tests should be a requirement before moving yards. Extra caution should be taken at shows – and perhaps in those with a high concentration of horses, strangles-negative serology should become a requirement.
New perspectives with DIVA recombining vaccines hitting the market look optimistic, but there is still a long way to go until proven efficacy in the field.
At this time, more investigation is needed to develop effective immunity in naive horses and to break the adaptive response host bacteria to avoid infected horses becoming carriers.
Acknowledgement
The author would like to thank Durham Equine Practice for allowing her to use the data in this article, for her years working there.
She also thanked colleagues who have shared opinions and suggested amendments to the article ahead of publication.
- Some drugs mentioned in this article are used under the cascade.
References
- Barquero N et al (2007). Evidence-based immunization in horses, Vet Clin North Am Equine Pract 23(2): 481-508.
- BEVA guidance and resources.
- Boehringer Academy.
- Boyle AG et al (2016). Streptococcus equi detection polymerase chain reaction assay for equine nasopharyngeal and guttural pouch wash samples, J Vet Intern Med 30(1): 276-281.
- Boyle AG et al (2017). Comparison of nasopharyngeal and guttural pouch specimens to determine the optimal sampling site to detect Streptococcus equi subsp equi carriers by DNA amplification, BMC Vet Res 13: 75.
- Boyle AG et al (2018). Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles – revised consensus statement, J Vet Intern Med 32(2): 633-647.
- Cordoni G et al (2015). Rapid diagnosis of strangles (Streptococcus equi subspecies equi) using PCR, Res Vet Sci 102: 162-166.
- Dong J et al (2019). An outbreak of strangles associated with a novel genotype of Streptococcus equi subspecies equi in donkeys in China during 2018, Equine Vet J 51(6): 743-748.
- Duran MC and, Goehring LS (2021). Equine strangles: an update on disease control and prevention, Austral J Vet Sci 53(1): 23-31.
- Durham AE and Kemp-Symonds J (2021). Failure of serological testing for antigens A and C of Streptococcus equi subspecies equi to identify guttural pouch carriers, Equine Vet J 53(1): 38-43.
- European Medicines Agency.
- HBLB (2022). International codes of practice: strangles.
- Mallicote M (2015). Update on Streptococcus equi subsp equi infections, Vet Clin North Am Equine Pract 31(1): 27-41.
- Morris ERA et al (2021). Differences in the genome, methylome, and transcriptome do not differentiate isolates of Streptococcus equi subsp equi from horses with acute clinical signs from isolates of inapparent carriers, PLOS One 16(6): e0252804.
- Newton JR (2011a). How to diagnose: strangles, Proc BEVA Congress, Liverpool.
- Newton JR (2011b). Strangles in the stud environment, Proc BEVA Congress, Liverpool.
- Slater J (2007). Bacterial infections of the respiratory tract. In McGorum BC, Dixon PM, Robinson NE and Schumacher J (eds), Equine Respiratory Medicine and Surgery, Saunders, Philadelphia: 327-353.
- Smith PA (2006). How to eliminate strangles infections caused by silent carriers, Proc Am Assoc Equine Pract Annual Conf, San Antonio.
- Surveillance of Equine Strangles.
- Sweeney CR et al (2005). Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles, J Vet Intern Med 19(1): 123-134.
- Tartor YH et al (2020). Novel Streptococcus equi strains causing strangles outbreaks in Arabian horses in Egypt, Transbound Emerg Dis 67(6): 2,455-2,466.
- The British Horse Society (2021). Strangles.
- Waller AS (2013). Strangles: taking steps towards eradication, Vet Microbiol 167(1-2): 50-60.
- Waller AS (2014). New perspectives for the diagnosis, control, treatment, and prevention of strangles in horses, Vet Clin North Am Equine Pract 30(3): 591-607.
- Willis AT et al (2021). Validation of a point-of-care polymerase chain reaction assay for detection of Streptococcus equi subspecies equi in rostral nasal swabs from horses with suspected strangle, Can Vet J 62(1): 51-54.
