20 May 2025
Fleur Whitlock BVetMed(Hons), MSc, MRCVS, Abbi McGlennon BSc(Hons), PhD and Richard Newton BVSc, MSc, PhD, FRCVS discuss this highly infectious disease, which can spread rapidly with a high morbidity rate, and the solutions available to curb the spread.


Image: encierro / Adobe Stock
Strangles is a bacterial equine disease reported in veterinary literature as early as the 13th and 17th centuries (Ruffus, 1251; Solleysel, 1664), and to this day remains a substantial risk to horses. The causative agent of the disease, Streptococcus equi, was identified in 1888 (Schütz, 1888) and is a host-restricted pathogen affecting horses, donkeys and mules (Holden et al, 2009).
Despite early historical references to the disease, a large-scale genetic analysis of internationally collated S equi strains found that the ancestor of contemporary strains dated back to the 19th century (Harris et al, 2015), providing evidence of a global population replacement of circulating S equi strains.
The timing of the shared ancestry corresponds to a period when horses were commonly used in warfare, enabling the mixing of horse populations and their pathogens on an international scale.
S equi is highly infectious, with high morbidity rates (Timoney, 1993), and can spread across premises rapidly if an infected horse is not identified and isolated upon presentation of clinical signs, or if biosecurity measures are insufficient.
Outbreaks affect multiple sectors of the UK equine industry, from competitions, riding schools, general-use yards as well as semi-feral herds of ponies that live in our national parks. No requirement exists to report a strangles outbreak or conduct contact tracing; therefore, examining how this pathogen transmits between horses is challenging and requires an “out-of-the-box” approach to begin understanding these dynamics.
S equi infections typically last around four to six weeks (Figure 1), with clinical signs progressing over the course of infection, including nasal discharge, pyrexia, lymphadenopathy culminating in abscessation and discharge, which can be more aggressive in naive horses (Boyle et al, 2018).
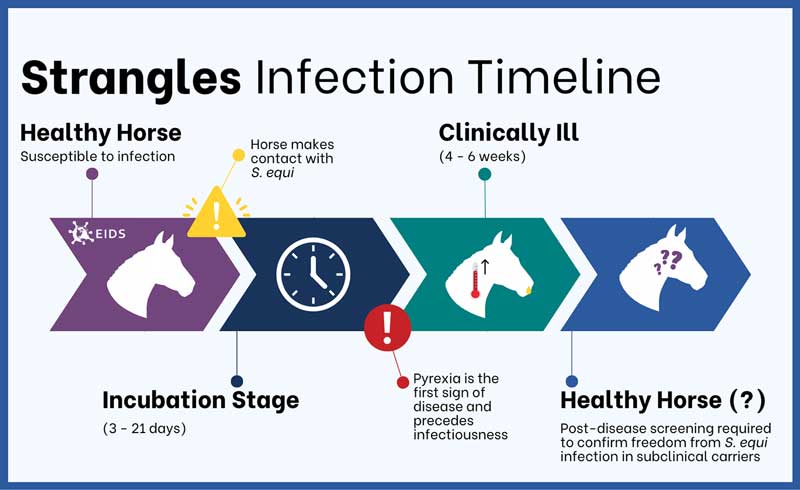
Strangles surveillance in the UK, based on laboratory detection of S equi between 2015-19, showed that nasal discharge and pyrexia were the most frequently observed clinical signs reported by veterinary surgeons at the time of sampling (McGlennon et al, 2021).
This indicates the need for equine veterinary surgeons to consider S equi in their testing when horses present with non-specific signs of respiratory infection, as these signs could be attributed to any of the UK endemic upper respiratory tract diseases.
The Surveillance of Equine Strangles (SES) network has been tracking strangles in the UK through real-time data from laboratory diagnoses since it was established in 2018 (McGlennon, 2019).
To date, 13 veterinary laboratories exist across the UK, contributing data when they have positive diagnoses of S equi infection based on agent detection methods such as culture, PCR or loop-mediated isothermal amplification.
Data reported, where available, includes horse signalment (age, sex, breed), premises type, clinical signs observed, reason for sampling and the geographical location based on the submitting veterinary practice. These data are then summarised and can be viewed via an online dashboard (www.equinesurveillance.org/ses).
Users can stay appraised of latest diagnoses of strangles in the UK including viewing diagnoses by regional location, a summary of clinical signs reported at the time of sampling and other information such as reasons for sampling. Data on diagnoses are available from 5 January 2015 up to the most recent diagnosis reported to SES in 2025, and users are able to change the date range for diagnoses based on their own interests.
During the authors’ PhD project up until 2022, SES was also functioning as an enhanced surveillance initiative where laboratories were also sending surplus clinical S equi samples to SES for whole genome sequencing and analysis. Enhanced surveillance integrates epidemiological and genomic data, and is a vital tool in monitoring infectious diseases (Figure 2); it represents a significant advancement in disease surveillance by offering nuanced insights into infectious disease dynamics.
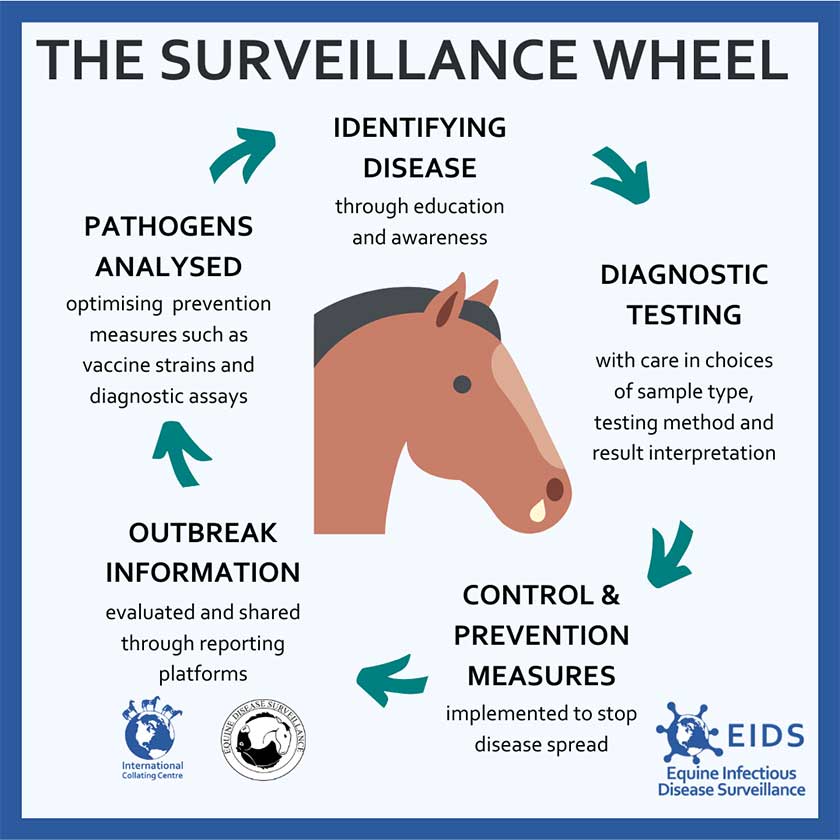
The research, undertaken at the RVC, investigated the epidemiology, genomics and transmission of S equi across the UK between 2016 and 2022 utilising data from SES, and is an important example of enhanced surveillance of an infectious equine pathogen (McGlennon, 2024).
Between 2016 and 2022, strangles diagnoses were widespread across the UK, including the Isle of Man and the Channel Islands, highlighting the continued endemicity of the disease (Figure 3). More than 60% of UK regions had one or more strangles diagnoses. Importantly, regions with no diagnoses reported cannot be considered free from disease, but rather show that no laboratory diagnoses were reported to SES.
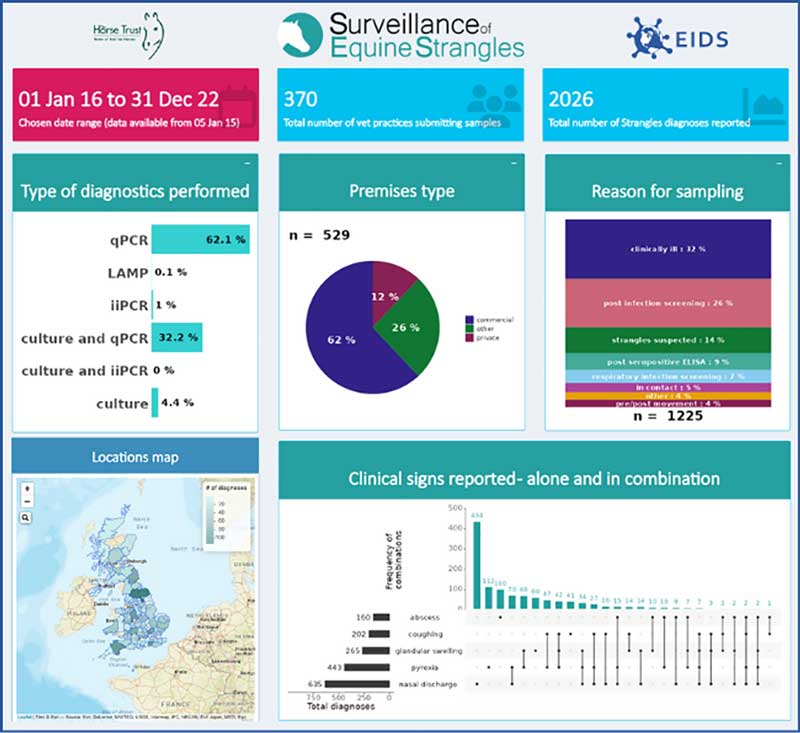
The majority of diagnoses were confirmed using molecular diagnostics rather than culture alone, with 32% of cases having both molecular and culture-based confirmation, while only 4% were identified through culture alone.
Sampling was conducted for various reasons (where provided by the submitting veterinary surgeon, n=1,225), including clinical illness, post-infection screening and subclinical screening. Encouragingly, 9% of cases (110 out of 1,225) were sampled following a positive serological test.
This allowed for potential actions such as isolating affected horses and treating infections, thereby reducing the risk of further transmission among populations and premises. However, it is important to note that these specific actions cannot be confirmed from the available data.
Genomic analysis of clinical S equi isolates recovered from horses between 2016 and 2022 revealed a relatively quick evolutionary change in the population structure of circulating strains of S equi in UK horses. Previous research has supported the hypothesis that subclinical carrier horses, harbouring established S equi strains, are the main driver of S equi endemicity (Pringle et al, 2019). In contrast, within the seven-year period examined as part of this PhD project, transmission was associated with a year-on-year change in relative proportions of S equi strains recovered from acute cases of disease or recently convalesced horses, which would not have been explained by persistence of established carrier strains driving strangles endemicity.
In addition, the use of transmission modelling combining genomic and epidemiological data provided further understanding of the dynamics of strangles outbreaks across the UK and revealed a number of direct transmission pairs (horses with laboratory-confirmed strangles diagnoses in the SES network where one horse was identified as the immediate source of infection for another) were linked between different regions of the UK.
This means that movements of horses over relatively long distances – typically for sales, breeding, competition or training events – are clearly influencing the spread of specific S equi strains widely across the UK and is clearly of national and probably international importance.
Furthermore, horse owners who experienced an outbreak between 2020-22 were surveyed regarding the management of their outbreak and many demonstrated a reactive implementation of biosecurity measures only in the face of a strangles outbreak, rather than before it. This is clearly suboptimal given that simple biosecurity practices such as regular hand washing and minimising contact with unknown horses can effectively reduce transmission of S equi, with little cost financially or in time to owners/handlers.
Moreover, when measures were applied during an outbreak, they were suboptimal for stringent and effective outbreak management with apparently minimal adoption of the recommended traffic light outbreak management system or regular temperature checking of horses on premises (Rendle et al, 2021). The findings from this research project have provided insights into where within the equine sector future awareness and education campaigns should be directed to yield the greatest benefit to horses and their owners. Therefore, new approaches into strangles prevention messaging need to be explored.
Preventing the spread of infectious diseases such as strangles requires a collaborative effort, and one effective approach involves four key actions: vaccinate, isolate, investigate and communicate – as Equine Infectious Disease Surveillance refers to them: “the four -ates” (Figure 4).

Isolating animals that are moving to new premises or returning after a period away is essential in preventing the introduction of infectious diseases. Investigating animals that show clinical signs allows for the confirmation of disease, and routine temperature checks serve as a simple yet effective early warning system for many infections.
Communication is equally important, as raising awareness when a disease is confirmed ensures that others can take appropriate action while also aiding in disease tracking and monitoring of vaccine effectiveness.
Vaccination is a crucial preventive measure, helping to protect against disease while its effectiveness is monitored through disease surveillance. Contributions to surveillance are, therefore, essential, with veterinary surgeons encouraged to test suspect cases and laboratories conducting testing urged to participate. SES is very appreciative to those already contributing the essential data that has informed this research.
By implementing these control and prevention measures, we can work together to reduce the impact of a number of infectious diseases, including strangles.
The availability of strangles vaccines varies globally, with different options accessible depending on geographical location. This discussion focuses on vaccinations available in the UK. In 2005, Equilis StrepE (MSD Animal Health UK), a submucosally administered, live attenuated vaccine, became commercially available in the UK (Kelly et al, 2006).
This vaccine provided immunity for a limited duration of three months. However, adverse reactions were reported in some horses (Kemp-Symonds et al, 2007).
Furthermore, horses vaccinated with live attenuated vaccines develop immune responses that are indistinguishable from those of horses that have experienced a clinical infection, which complicates diagnostic testing and outbreak investigations. It is important to note that Equilis StrepE is no longer available in the UK.
The currently available vaccine in the UK is Strangvac (Intervacc), which is based on a targeted selection of recombinant S equi proteins (Robinson et al, 2020). As this vaccine does not contain live bacteria or bacterial DNA and the S equi proteins have been specifically selected, it does not interfere with diagnostic quantitative PCR or indirect ELISA assays. This means Strangvac has differentiating infected from vaccinated animals (DIVA) capability. For example, it is possible to “differentiate infected from vaccinated animals”, which is important because it allows use of the vaccine alongside diagnostic testing in managing active outbreaks of strangles – something that has not been possible with previous S equi vaccines.
Strangvac was released for commercial use in the UK in August 2022, following its approval for use across the EU, Norway and Iceland in autumn 2021, and in Sweden in early 2022. Strangvac is recommended for horses at high risk of exposure.
Since its introduction, a group of leading strangles experts met in Sweden in late 2023 to review experiences with Strangvac’s use across Europe (Rendle et al, 2024). Their findings highlighted key considerations for vaccination strategies, including the recommendation that while universal vaccination against strangles is theoretically beneficial, achieving full compliance is unlikely. Consequently, the experts have identified specific scenarios and premises where vaccination should be particularly encouraged. As an example, equestrian businesses where an outbreak would threaten commercial viability and disrupt schedules should prioritise vaccination.
Similarly, horses attending amateur competitions with suboptimal biosecurity measures would benefit from immunisation. Studs that accept walk-in mares, as well as the mares visiting them, are also considered a high priority for vaccination. Additionally, premises that meet any of the following criteria should strongly consider implementing vaccination protocols: frequent arrival of new horses, high numbers of visiting horses, significant populations of foals and young horses, horses under mixed ownership, or generally premises with poor biosecurity standards.
These measures aim to reduce the risk of strangles outbreaks and enhance equine health management across the sector.
The application of vaccination in real-world settings presents additional challenges due to the unpredictable and likely variable exposure of horses to S equi, as well as the differing immune status of individual animals (Rendle et al, 2024).
However, the benefits of Strangvac vaccination are increasingly being observed in strangles outbreaks across equestrian properties in various European countries where the vaccine has been adopted. In Sweden, an outbreak on a partially vaccinated yard demonstrated a significant difference in infection rates between vaccinated and unvaccinated horses.
Clinical signs were observed in only 2 out of 20 vaccinated horses, compared to 48 out of 65 unvaccinated horses (Gröndahl, unpublished observations). These findings highlight the potential effectiveness of Strangvac in reducing the impact of strangles outbreaks.
Vaccination with Strangvac can also be used in an outbreak management plan that utilises the traffic light system for separating clinical cases (red group), healthy but potentially exposed animals (amber group), and healthy unexposed animals (green group; Figure 5). The following recommendations are from Rendle et al (2024).
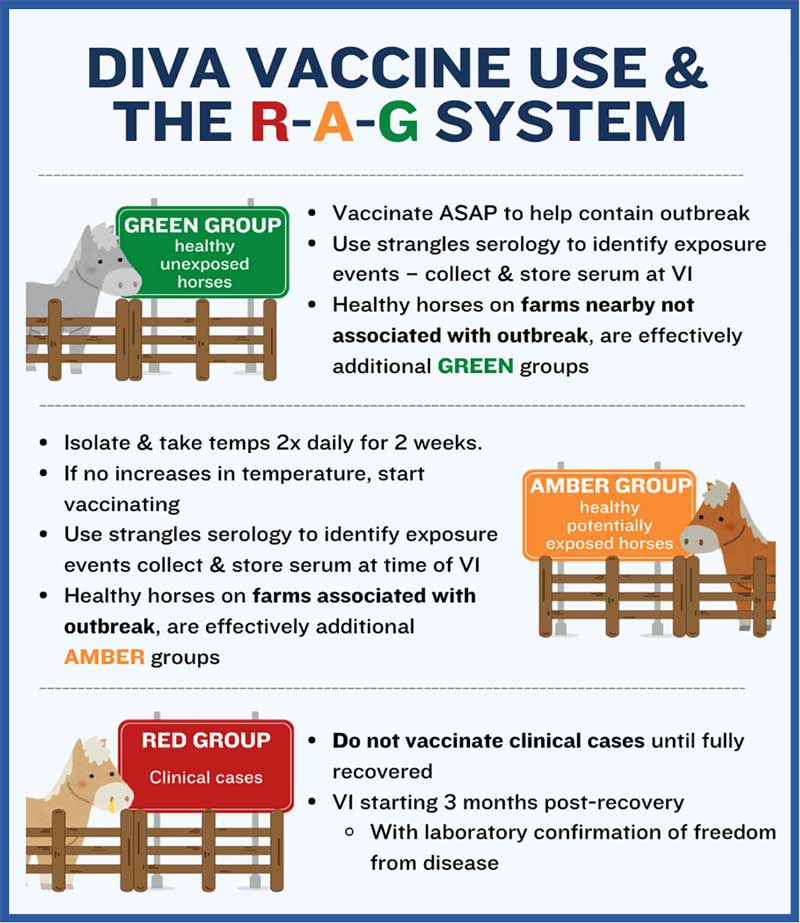
The green group consists of healthy, unexposed animals. Vaccination in this group should begin as soon as possible to help contain the outbreak and reduce the risk of further spread. Strangles serology can be used to identify any exposure events, with serum samples collected and stored at the time of the first vaccination. Additionally, healthy horses on nearby farms that are not directly linked to the outbreak should also be considered part of the green group and vaccinated accordingly.
The amber group includes healthy horses that may have been exposed to infection. These horses should be isolated and monitored closely, with temperatures taken twice daily for two weeks. If no increase in temperature is observed, the vaccination protocol can begin.
Strangles serology should be used to detect any exposure, with serum collected and stored at the time of the first vaccination. Healthy horses on farms associated with the outbreak should also be classified as amber group animals and managed in the same manner.
It should be noted that since the review meeting in late 2023, some experts are now advocating the use of Strangvac immediately in amber group horses, rather than only after a period of two weeks isolation with clinical monitoring.
Some caution is advised with this approach, as Strangvac-vaccinated horses may spike raised temperatures, which can be an early clinical sign in strangles. It may, therefore, be challenging to differentiate a vaccine-induced response from an infectious case, potentially leading to incorrect outbreak management decisions and further disease spread.
The red group consists of clinical cases. Vaccination should not be administered to horses showing signs of strangles until they have fully recovered.
The first dose of the vaccine should be given no earlier than three months post-recovery, with laboratory confirmation (through guttural pouch lavage, tested by PCR) required to ensure the horse is free from disease before vaccination begins. Healthy carriers (visibly well horses that harbour S equi in their guttural pouches) can be vaccinated, and early feedback suggests that vaccination may help to resolve carrier status; however, the need to collect further data exists before this is proven.
To conclude, strangles remains endemic in the UK, posing a persistent challenge to equine health.
Recent findings suggest that acute and recently convalesced horses play a more significant role in the transmission of S equi than previously understood, although long-term carriers are still important to the disease’s persistence.
Fortunately, straightforward and effective solutions are available to curb its spread, including proper biosecurity and hygiene practices.
Additionally, vaccination with Strangvac represents a major advancement in the effort to control strangles, with success reported from multiple countries adopting its use.
These recent research findings and perspectives from colleagues in Europe offer hope for more comprehensive prevention strategies to be utilised by yard and horse owners in the UK moving forward.
