1 Aug 2019
Tendon injuries in horses: treatment and management
Annamaria Nagy discusses the therapy options and management protocols for patients that have this issue due to overstrain.

Image: © Rade Lukovic / Adobe Stock

Tendon injuries are common in all types of horses. Although a higher prevalence occurs in performance horses undergoing strenuous exercise, these injuries are also diagnosed in leisure horses.
Injuries can occur by trauma or overstrain; the latter will be discussed in this article.
The tendons’ mechanical characteristics and internal architecture depend on their function. Energy-storing tendons, such as the superficial digital flexor tendon (SDFT), have different mechanical and internal architecture from positional tendons, such as the common digital extensor tendon. Overstrain injuries may result from a sudden overloading of the tendon.
However, increasing evidence suggests a degenerative process precedes clinical injury, especially in the palmar metacarpal soft tissue structures (Birch et al, 2014). Gross and microscopic lesions have been documented on postmortem examination of tendons of horses with no clinical signs of tendon injury (Goodship et al, 1994).
In many horses, tendon lesions are identified bilaterally, even if clinical signs are restricted to one limb. Epidemiological and experimental studies have demonstrated associations between tendon injury, and age and exercise (Wilson et al, 1996; Smith et al, 2002). It has been suggested overload and vascular disruption may initiate inflammation.
Unlike bones and muscles that respond to training and exercise, tendons can only adapt to exercise in young foals. By the age of 11 months, the biomechanical properties of the tendon are similar to those of mature horses (van Weeren and Barneveld, 1999).
Tendons have limited intrinsic repair capacity and repaired tendon tissue is almost always inferior. Following the initial haemorrhage and inflammatory phase – and within a few days – the reparative phase begins, with pronounced angiogenesis and scar tissue formation. This tissue has a much higher type-three collagen to type-one collagen ratio than normal tendon tissue.
During the remodelling phase, some of the type-three collagen is replaced by type-one collagen, but the replacement is incomplete. The length of these phases is variable, which can make appropriate timing of specific treatments challenging. The repaired tendon (scar tissue) is less stiff as a material than tendon, but as large amounts of scar tissue are formed, the scarred tendon is often stiffer as a structure than the original tendon.
Incomplete resolution of inflammation in advanced disease indicates failure of the tendon to return to normal and development of chronic inflammation (Dakin, 2017). Aged horses have a reduced ability to respond to inflammation, which may explain their reduced efficacy of tendon repair, and may present a potential mechanism for chronic inflammation and re-injury (Dakin, 2017).
As injuries most commonly involve – and most research has focused on – the SDFT, this article will discuss treatment and management options of SDFT injuries in the metacarpal region (Figure 1).
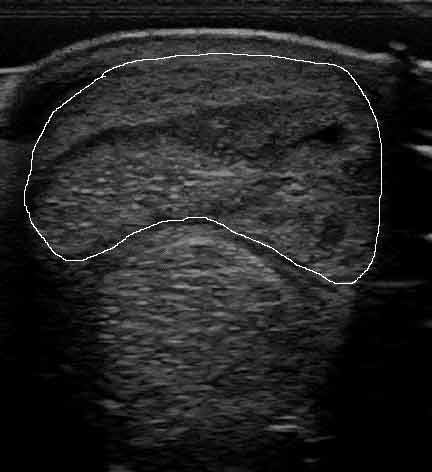
Treatment options
Over the years, many treatments have been tried and new modalities are continuously appearing – confirming that, to date, a successful treatment with convincing long-term results has not been identified. Treatment modalities to be applied should have a rationale for addressing various phases of the healing process.
In the acute, inflammatory phase, the aim is to minimise inflammation and limit proteolytic enzyme activity that continue to destroy tendon tissue. Cryotherapy and anti-inflammatory medication are important.
Cryotherapy can be applied in many ways – including icing, application of ice boots, a machine using a wrap that delivers dry cold and compression, or in a cold water spa. Compression, by bandaging, can help reduce swelling.
Systemic corticosteroids should only be applied in the first 24 to 48 hours because they can later inhibit the second phase of fibroplasia, and intralesional corticosteroids should be avoided because of the risk of calcification.
Complete inflammatory blockade may be potentially deleterious because inflammation has beneficial components, and is necessary for the debridement of tissues and recruitment of immune cells to the injured area (Dakin, 2017).
Prolonged use of cyclooxygenase-2 selective NSAIDs should be avoided, because they have been shown to reduce endogenous resolution responses and prolonged use has been associated with a deleterious effect on collagen synthesis, which may impair the tendon’s ability to heal (Dakin, 2017).
Percutaneous tendon splitting was thought to release pressure from haemorrhage and promote revascularisation of core lesions (Witte et al, 2016), but convincing benefits of this treatment have not been shown in studies and the induced surgical trauma is of concern.
Another surgical treatment, sometimes applied at the same time as tendon splitting, is desmotomy of the accessory ligament of the SDFT. The aim is to reduce peak strains in the SDFT by increasing the work of the SDF muscle and lengthening the check ligament-tendon unit (Hu and Bramlage, 2014). One study reported success (Bramlage et al, 1988); however, it was not confirmed in further studies and increased incidence of suspensory desmitis following surgery has been reported (Hu and Bramlage, 2014).
Extracorporeal shockwave therapy has been used, but with only transient improvement at best in most cases, most likely due to its analgesic effect. It has been shown to increase tenocyte metabolism in vitro (Bosch et al, 2009) and was, therefore, hypothesised to stimulate remodelling of scar tissue, which could be beneficial in chronic injuries. However, this has not been confirmed in clinical studies.
Intralesional treatment is most commonly applied in the subacute or fibroblastic phase. A plethora of treatment options are available, but the choice is more guided by the clinician’s experience and anecdotal information than evidence-based data.
The first intralesional treatments included hyaluronic acid and polysulphated glycosaminoglycans, with equivocal results. Hyaluronic acid may help reduce adhesion formation in case of intrathecal injuries. β-aminopropionitrile fumarate was used with the aim of improving longitudinal fibre alignment and improving the quality of the scar tissue. β-aminopropionitrile fumarate is a lysyl oxidase inhibitor that prevents cross-linking of collagen molecules.
It was hypothesised delaying collagen cross-linking would allow time for the fibres to align correctly under the stimulus of controlled exercise (Dahlgren et al, 2001). However, additionally to lack of efficacy in experimental studies, it has been suggested delayed cross-linking can reduce the tensile strength of tendons and, therefore, increase the risk of catastrophic fracture during rehabilitation (Dahlgren et al, 2001).
Some clinicians found β-aminopropionitrile fumarate a more effective treatment than other available options, but often encountered re-injury in the contralateral limb of horses with unilateral injuries (Dyson, personal communication).
Regenerative treatments
The intralesional anabolic growth factors (insulin-like growth factor, recombinant equine growth hormone and transforming growth factor-β) have been explored empirically and in experimental settings (Dahlgren et al, 2001; Dowling et al, 2002), but their safety and efficacy have not been assessed in clinical studies.
Platelet-rich plasma (PRP) is frequently used, despite the lack of robust clinical evidence of its efficacy; probably because of its ready availability and relative low cost compared to stem cell therapy. PRP is rich in anabolic growth factors that can stimulate tenocyte proliferation and matrix synthesis.
PRP is prepared from autologous blood using commercially available kits. The composition of PRP can be variable, which can contribute to the less favourable results of clinical trials (Geburek et al, 2016) than those of experimental studies. PRP preparations also contain leukocytes that have a proinflammatory effect deleterious to tendon healing (Smith et al, 2014). In an in vitro study, extracorporeal shockwave therapy increased growth factor release from PRP – suggesting the combination of these two modalities may have a synergetic effect (Seabaugh et al, 2017); however, this is yet to be confirmed in clinical studies.
Autologous mesenchymal stem cells (MSCs) have been advocated to create a repair superior to scar tissue, by differentiating into tenocytes and regenerating matrix (Birch et al, 2014). It is not certain whether the therapeutic effect is due to the injected MSCs’ differentiation into tenocytes or due to indirect paracrine effects (Geburek et al, 2017).
Although fat-derived MSCs have been explored, bone marrow-derived MSCs are more commonly used in both clinical and experimental settings. Bone marrow is usually harvested from the sternum (Figure 2; an alternative site is the tuber coxae), and cells are cultured for two to three weeks until a cell count of between 10 million and 20 million is achieved, although it can take longer in older horses.
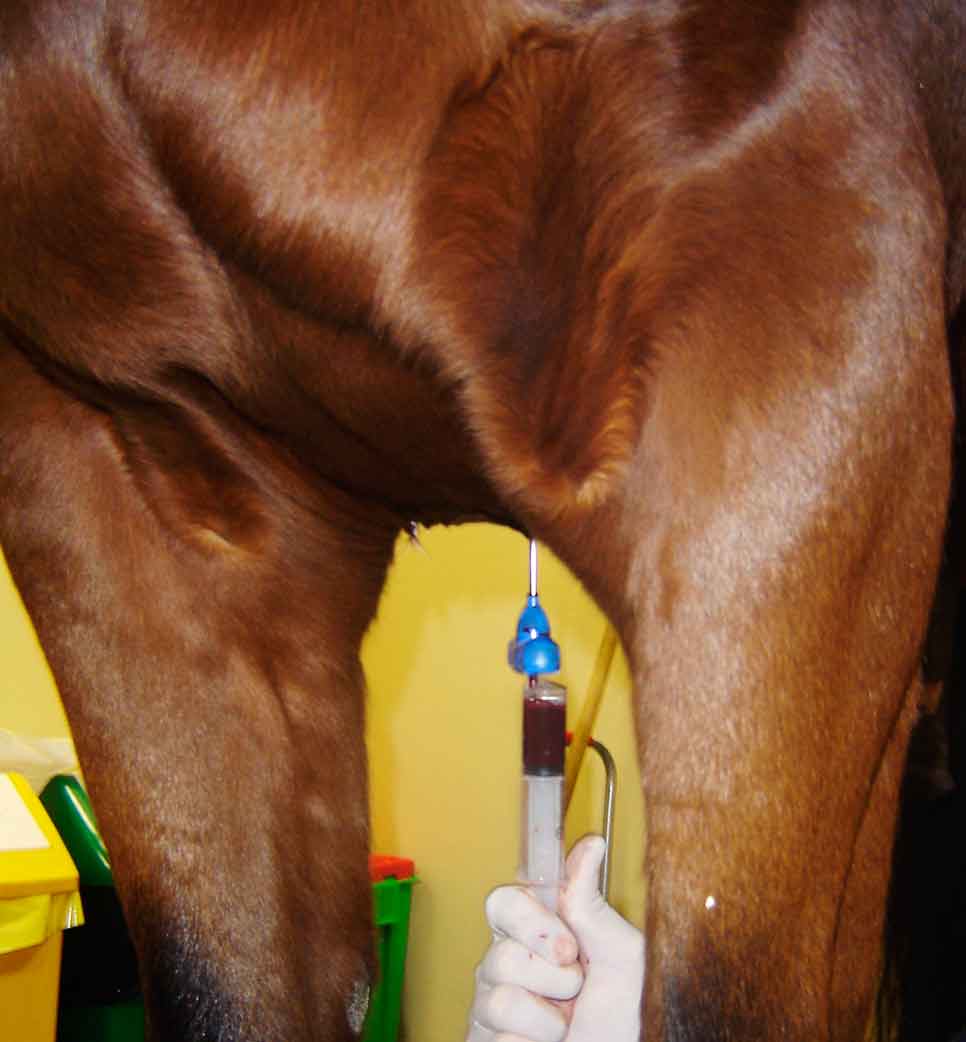
Injection of stem cells is ideally performed after the inflammatory phase, but before scar tissue forms. It has been shown the tenogenic properties of mesenchymal progenitor cells are compromised in an inflammatory environment (Brandt et al, 2018) – supporting the practice that injection of stem cells in the acute phase should be avoided, which is not a likely problem in practice anyway due to the time taken to culture a sufficient number of MSCs.
An early study on naturally occurring injuries showed promising results. The re-injury rate in a population of 141 National Hunt racehorses whose SDFT lesions were treated with bone marrow-derived MSCs was 27% after three years – considerably lower than previously published results (Godwin et al, 2012).
A more recent study compared the responses of 95 performance horses of various disciplines with tendon or ligament injuries, treated with either autologous bone marrow or allogenic amnion-derived stem cells (treatment was randomly assigned; Lange-Consiglio et al, 2013). Horses were followed for two years from return to full work. Horses treated with amnion-derived stem cells had lower re-injury rate (4% versus 23%) and returned to work earlier (4 to 5 months post-treatment) than horses treated with bone marrow-derived stem cells (4 to 12 months).
Amniotic-derived stem cells could be an exciting treatment option as products could be available off the shelf – eliminating harvest-related risk and waiting time of cell culture. However, technical difficulties – such as cryopreservation – are yet to be resolved before such a product becomes available.
Intralesional gene therapy – using plasmid DNA encoding vascular endothelial and fibroblast growth factors – has been trialled, with 8 of 10 horses returning to pre-injury level after 2 to 6 months and remaining in full work until the end of the follow-up period of 12 months (Kovac et al, 2017).
These promising results have to be confirmed in other studies, with a longer follow-up and the mechanism of treatment investigated in laboratory studies.
Regenerative (high-power) laser therapy is becoming a popular treatment of choice; however, to date, little evidence of its efficacy exists. A clinical study of 150 sports horses with tendon or ligament injury showed more rapid improvement in lameness and ultrasonographic scores than previously described treatments (Pluim et al, 2018). Long-term (24 months) follow-up was only available for 22% of horses.
Management protocols
Despite the high prevalence of tendon injuries, rehabilitation exercise and management protocols are purely empirical, and based on anecdotal information and clinicians’ experiences.
It is generally considered tendon injuries require a prolonged time to heal – return to full athletic function should not be expected before 6 to 12 months, depending on the location and severity of the injury. During rehabilitation, horses should be monitored regularly – clinically and ultrasonographically, particularly before exercise is increased. Ultrasonographic examination should include cross-sectional area measurements of the SDFT to assess the tendon’s response to increased loading.
Horses are generally confined to box rest and controlled walking exercise in the first few months of rehabilitation. Walking exercise should begin immediately and the majority of horses can be walked for up to 30 minutes a day in one or two blocks.
Horses with severe injuries may need to walk for a shorter time initially during the inflammatory phase. The only contraindication for early walking exercise is an injury so severe that rupture of the tendon is a realistic risk.
Where horses are walked is usually driven by the facilities and the horse’s temperament. Horses can be walked in-hand, on a horse walker or on a dry treadmill. In some horses, ridden walking exercise may be the best option if it is difficult to control in hand. The rider should be lightweight to minimise added loading of the SDFT.
Water treadmill can be used from the second month after injury, with a low water level (foot height) and at a relatively low speed (Nankervis et al, 2017). Walking is usually increased in the second month and, depending on clinical and ultrasonographic improvement, trotting is introduced after three to five months.
Following injury, proprioception can be altered by damage to the connective tissue and sensory neural tissue in joint capsules, ligaments, musculotendinous junction and muscle spindles. Therefore, proprioceptive retraining – including pole work taping and use of lightweight chains in the pastern – can be beneficial as part of the rehabilitation (Goff, 2014).
Specific shoes have been recommended (for example, flat, thin shoes with wide toe and narrow heels; Denoix et al, 2005), but shoeing has not been shown to alter the healing process or outcome. Efforts should, however, be made to improve any foot imbalance and abnormal foot conformation, if possible.
The SDFT is functionally analogous to the human Achilles tendon, and SDFT lesions often serve as a model for Achilles and rotator cuff tendon injury. It appears human patients are introduced to various forms or exercises and can return to full function earlier than clinicians generally advise for horses – and more focus is placed on rehabilitation exercise than medical treatment. Humans are advised to exercise to a pain threshold in a graded way, which cannot be done in horses. Undoubtedly, alternative exercise – such as swimming and cycling in human patients – is much more difficult to apply in horses, but increasing use of physiotherapy exercises, water treadmill and swimming should perhaps be explored.
- Some drugs mentioned are not licensed for veterinary use.
References
- Birch HL et al (2014). Tendon and ligament pathology. In Hinchcliff KW, Geor RJ and Kaneps AJ (eds), Equine Sports Medicine and Surgery (2nd edn), Saunders Elsevier, Philadelphia: 167-188.
- Bosch G et al (2009). The effect of focused extracorporeal shock wave therapy on collagen matrix and gene expression in normal tendons and ligaments, Equine Vet J 41(4): 335-341.
- Bramlage LR et al (1988). Long-term effects of surgical treatment of superficial digital flexor tendinitis by superior check ligament desmotomy, Proc Am Assoc Equine Pract 34: 655-656.
- Brandt L et al (2018). Tenogenic properties of mesenchymal progenitor cells are compromised in an inflammatory environment, Int J Mol Sci 19(9): 2,549.
- Dahlgren LA et al (2001). Effects of ß-aminopropionitrile on equine tendon metabolism in vitro and on effects of insulin-like growth factor-I on matrix production by equine tenocytes, Am J Vet Res 62(10): 1,557-1,562.
- Dakin SG (2017). A review of the healing processes in equine superficial digital flexor tendinopathy, Equine Vet Educ 29(9): 516-520.
- Denoix JM et al (2005). Corrective shoeing of equine distal limb injuries, Proc 9th Congress Equine Med Surg, Geneva: 79-82.
- Dowling BA et al (2002). Recombinant equine growth hormone does not affect in vitro biomechanical properties of equine superficial digital flexor tendon, Vet Surg 31(4): 325-330.
- Geburek F et al (2016). Effect of intralesional platelet-rich plasma (PRP) treatment on clinical and ultrasonographic parameters in equine naturally occurring superficial digital flexor tendinopathies – a randomized prospective controlled clinical trial, BMC Vet Res 12(1): 191.
- Geburek F et al (2017). Effect of single intralesional treatment of surgically induced equine superficial digital flexor tendon core lesions with adipose-derived mesenchymal stromal cells: a controlled experimental trial, Stem Cell Res Ther 8: 129.
- Godwin EE et al (2012). Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon, Equine Vet J 44(1): 25-32.
- Goff L (2014). Manual therapy and exercise for athletic horses. In Hinchcliff KW, Geor RJ and Kaneps AJ (eds), Equine Sports Medicine and Surgery (2nd edn), Saunders Elsevier, Philadelphia: 1,217-1,224.
- Goodship AE et al (1994). The pathobiology and repair of tendon and ligament injury, Vet Clin North Am Equine Pract 10(2): 323-349.
- Hu AJ and Bramlage LR (2014). Racing performance of Thoroughbreds with superficial digital flexor tendonitis treated with desmotomy of the accessory ligament of the superficial digital flexor tendon: 332 cases (1989-2003), J Am Vet Med Assoc 244(12): 1,441-1,448.
- Lange-Consiglio A et al (2013). Investigating the efficacy of amnion-derived compared with bone marrow-derived mesenchymal stromal cells in equine tendon and ligament injuries, Cytotherapy 15(8): 1,011-1,020.
- Kovac M et al (2017). Gene therapy using plasmid DNA encoding vascular endothelial growth factor 164 and fibroblast growth factor 2 genes for the treatment of horse tendinitis and desmitis: case reports, Front Vet Sci 4: 168.
- Nankervis KJ et al (2017). The use of treadmills within the rehabilitation of horses, J Equine Vet Sci 53: 108-115.
- Pluim M et al (2018). Short- and long-term follow up of 150 sports horses diagnosed with tendinopathy or desmopathy by ultrasonographic examination and treated with high-power laser therapy, Res Vet Sci 119: 232-238.
- Seabaugh KA et al (2017). Extracorporeal shockwave therapy increases growth factor release from equine platelet-rich plasma in vitro, Front Vet Sci 4: 205.
- Smith RK et al (2002). The influence of ageing and exercise on tendon growth and degeneration – hypotheses for the initiation and prevention of strain-induced tendinopathies, Comp Biochem Physiol A Mol Integr Physiol 133(4): 1,039-1,050.
- Smith RK et al (2014). Advances in the understanding of tendinopathies: a report on the Second Havemeyer Workshop on equine tendon disease, Equine Vet J 46(1): 4-9.
- van Weeren PR and Barneveld A (1999). Introduction: study design to evaluate the influence of exercise on the development of the musculoskeletal system of foals up to age 11 months, Equine Vet J 31(Suppl 31): 4-8.
- Wilson JH et al (1996). Equine soft tissue injuries associated with racing: descriptive statistics from American racetracks, Proc Dubai Int Equine Symp: 1-22.
- Witte S et al (2016). Comparison of treatment outcomes for superficial flexor tendonitis in National Hunt racehorses, Vet J 216: 157-163.
