25 Feb 2025
Jacqui Matthews BVMS, PhD, FRSE, FRCVS says that with no new treatments on the horizon, it’s vital we continue to ensure the ones we have continue to do the job. The author looks at all the important aspects vets should consider.

Figure 1. Tools The “What’s your worm risk?” tool helps evaluate risk.
Parasitic worms are common in horses. The most prevalent are the cyathostomins (small redworms) and the tapeworm, Anoplocephala perfoliata.
Large strongyle infections are rarely encountered in horses exposed to long-term, broad-spectrum anthelmintics and the pinworm, Oxyrus equi, is an occasional issue in some herds. On breeding farms, the ascarid Parascaris species commonly affects foals, causing disease when large burdens build up.
In all cases, except large strongyles, anthelmintic resistance has been reported. With no new anthelmintics on the horizon, resistance poses a significant threat to horse health.
In the UK, new guidelines aim to promote sustainable control methods. BEVA introduced the “ProtectMEtoo” toolkit (beva.org.uk) in spring 2024 and the sector-wide “Controlling Antiparasitic Resistance in Equines Responsibly” (CANTER) guidelines (canterforhorses.org.uk) were released in October 2024.
Both resources emphasise the importance of worm control planning based on risk assessment and testing to inform treatment decisions.
Risk assessments should be conducted to establish individual or group risk of infection. Factors to consider include:
Conducting a risk assessment is essential for individual and group-kept horses. It should evaluate each horse’s susceptibility to different worms, expected environmental transmission based on management practices and recent test results. Key components of the assessment should include:
Horse age. Younger horses (younger than five years) are more prone to infection and higher burdens, increasing the risk for the entire paddock. Older horses may be at higher risk, especially those with pituitary pars intermedia dysfunction. Consider the age dynamics in each grazing group.
Grazing pattern and paddock management. Horses that graze for longer periods, especially year-round grazers, are at higher risk of infection. Important paddock management factors include:
Introduction of new horses/quarantine procedures. New arrivals can introduce new/resistant worms. Consider the effectiveness and consistency of quarantine measures applied.
Test results. Testing is essential for understanding infection levels in individuals and groups.
It is important to consider potential risks if other horses in the grazing area are not tested or their results are unknown.
Using the information collected, actions are then implemented to reduce the risk of infection, parasite-associated disease and resistance.
Risk can fluctuate based on individual factors (such as age or concurrent disease), season, management practices and test results over time, so horses at high risk should be reassessed every three months, those at moderate risk every six months, and those at low risk annually. Adjustments in management and testing frequency are required if circumstances change.
Assessing risk can be complex, as management practices and test outcomes may overlap across risk levels. To aid in this process, a free, user-friendly online tool, “What’s your worm risk?” (Figure 1), is available (whatsyourwormrisk.com). This tool evaluates risk levels based on nine key questions and is suitable for all age categories except foals.
Worm control should prioritise strategies that lower parasite levels on pasture to reduce host burdens and minimise treatment needs, thereby decreasing resistance risk.
Key practices include regular dung removal, maintaining low stocking densities, resting paddocks and using cattle/sheep to graze equine paddocks.
Many adult horses on well-managed paddocks have low parasite burdens due to a negative binomial distribution, where around 20% of the population carries approximately 80% of the parasites. Testing such populations to guide treatment decisions can significantly reduce anthelmintic use.
Two types of tests are useful for informing worming decisions:
Faecal egg count (FEC) tests estimate worm egg shedding, helping target treatments to reduce pasture contamination. These should also be used to assess anthelmintic effectiveness.
Antibody tests measure specific antibodies that correlate with infection level. Blood tests can detect tapeworm and small redworm infections, while a tapeworm saliva test enables owner sampling.
In well-managed settings, fewer horses test positive and require treatment, reducing resistance risk. In poorly managed environments, more horses get infected, increasing treatment needs and resistance risk. Figure 2 summarises relationships between management, testing, treatment frequency and resistance selection.
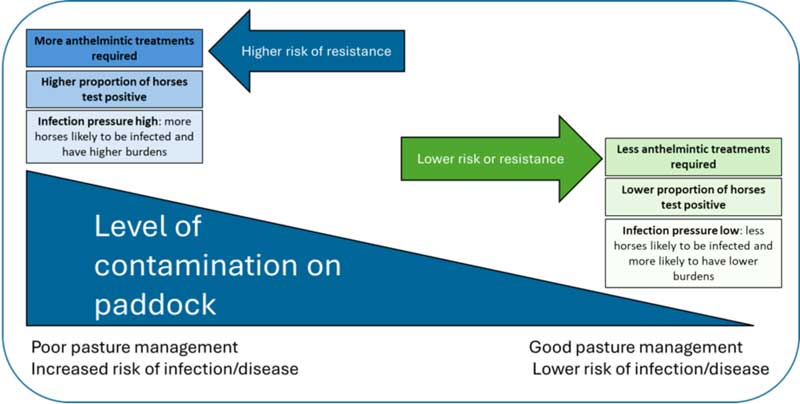
To reduce infection risk, consider management and environmental factors that affect worm development and survival. In the UK, the highest transmission risk is from mid-spring to late summer, making it vital to optimise management practices during this period.
To lower infection risk, fully remove faeces from paddocks, placing it well away from grazing areas to avoid third-stage larvae (L3) and oribatid mites from returning. Strongyle eggs develop to L3 in 3 to 4 days at 25°C to 33°C1, making timely removal essential. Twice-weekly removal significantly reduces strongyle L3 contamination and increases grazing areas2, while the same removal frequency significantly decreased the FEC of equids grazing managed paddocks compared to those where dung was not removed3.
Time spent on pasture, season and management all play crucial roles in infection risk. High stocking densities elevate risk4. Significantly higher strongyle FEC is found in horses kept at high densities (more than 30 horses per hectare) compared to those kept at lower densities. Stocking rates of 1 horse to 1 to 1.5 acres are recommended.
Strongyle larvae and unhatched eggs survive in cold conditions5, so stages deposited in one season can remain viable into the next. Resting pastures until mid-way through the next year can reduce infection risk, because L3 do not feed and have limited energy reserves depleting rapidly at higher temperatures6.
Complete eradication may take two years7. Year-round grazing increases contamination, evidenced by strongyle egg-shedding patterns during winter8. Little is known about the persistence/survival of cysticercoid-infected mites and ascarid eggs in UK conditions, so assume that these will persist on contaminated paddocks from year to year.
Alternate grazing of horses and ruminants can reduce worm transmission, as most parasites do not cross between these hosts; ponies grazing previously sheep-grazed paddocks were found to harbour substantially lower cyathostomin burdens than those on permanent equine paddocks9. Fasciola hepatica completes its life cycle in ruminants and horses and is pathogenic10. Testing ruminants for fluke infection is recommended before introduction to equine paddocks.
Targeting treatments based on strongyle egg shedding significantly reduces anthelmintic use11. In managed populations, 20% to 30% of horses shed approximately 80% of the total eggs excreted8,11.
Egg shedding varies with management, age, time since the last treatment and season, being typically higher in summer and lower in winter in the UK12,13. Young horses (aged one to four years) generally have higher FECs, while seniors (20 years plus) show increased FECs14.
Test every 8 to 12 weeks (more frequently in high-risk groups) and use a treatment threshold of 200 to 500 eggs per gram.
FECs do not correlate linearly with total worm counts15, so cannot reliably estimate burdens or disease risk. High-risk horses require special consideration; for example, cyathostomin larvicidal treatments are recommended in autumn/winter when mucosal larvae are more likely to be abundant16. For low-risk horses, the small redworm blood test17 can help evaluate cyathostomin burdens to avoid unnecessary treatments.
FEC tests are not reliable for detecting tapeworm infections due to low sensitivity, especially an inability to account for immature/sterile worms18,19.
Antibody tests measuring A perfoliata-specific IgG(T) are more sensitive20,21. Tapeworm testing is available in blood and saliva formats, with results reported as “low”, “borderline” or “moderate/high”, with horses having “low” results not recommended for treatment.
Two-thirds of more than 200,000 tapeworm saliva test results have been shown to fall within this category, leading to substantial reductions in anti-cestode treatments in the UK in the past decade22.
Horses are at risk of year-round tapeworm infection23. Those assessed as moderate to high risk should be tested twice a year (spring and autumn). Those assessed as low risk should be tested once a year (spring or autumn).
Spring testing enables targeting of infected horses to reduce egg shedding at a time when intermediate host oribatid mites are more likely to be active on paddocks. Autumn testing identifies horses with burdens that may put them at risk of colic. Since anthelmintics for tapeworms have a short half-life, it is important to appropriately manage paddocks to reduce reinfection risks.
Reducing the frequency of anthelmintic treatments may increase the prevalence of Strongylus vulgaris24. To address this, conduct annual monitoring where macrocyclic lactone treatments have not been administered for a time.
To identify infection, faecal culture should be undertaken in specialist laboratories to isolate L3, which can be differentiated from cyathostomin L3.