19 Jun 2017
Sonya Miles describes the case of a panther chameleon that presented with these symptoms, along with grip weakness.

Figure 4. The chameleon with placement of the pharyngostomy tube.
Reptiles are increasing in popularity and, as such, are becoming more frequently presented to vets with a myriad of conditions – most often as a result of environmental deficiencies. Therefore, general knowledge of the correct husbandry conditions specific to the species is essential when seeing these reptiles in practice. Urinary disorders in reptiles are a common occurrence (Funk, 2006), with signs often being non-specific, such as anorexia, behavioural changes and gout (Miller, 1998).
Anorexia, however, is a common symptom of various infectious and metabolic diseases, as well as a result of substandard environmental conditions (Funk, 2006). Making a quick diagnosis and eliminating the underlying cause, if possible – as quickly as possible – is essential for successful treatment Miller, 1998). This should always be combined with a reassessment and subsequent improvements of environmental conditions.
A four-year-old entire male panther chameleon (Furcifer pardalis) was presented with a one-week history of anorexia; submandibular, hindlimb and cask oedema; and weakness in grip in all four limbs and his tail (Figure 1). The chameleon’s owners had noticed he had been falling from his branches on numerous occasions.

The chameleon was provided with limited opportunities to hydrate (infrequent spraying and no permanent dripper unit), a significantly older than recommended ultraviolet light source (three years old when it should be changed every six months to a year depending on the brand being provided) and a high-protein diet with no calcium supplementation. His temperatures were also inadequate, as was the size and type of enclosure.
On initial examination, the chameleon was in poor body condition, weighing 200g. He was ambulatory, but did have a weaker than normal grip and obvious hindlimb, cask and submandibular swelling.
Assessment of his cardiovascular system with a Doppler unit was within normal limits, as was his respiration rate and effort. Coelomic palpation was also within normal limits.
The chameleon was restrained and placed in dorsal recumbency, and a 1in, 25-gauge needle attached to a 1ml syringe was sterilely inserted into the ventral coccygeal vein, at a 45° angle, to obtain a blood sample. Care was taken to avoid the chameleon’s hemipenes (Reavill and Shmidt, 2010).
Pressure was then applied to the collection site post-sampling. The 1ml of blood obtained was placed into a heparinised collection tube and blood smears were made.
The blood was sent off for external haematological and biochemical analysis (Tables 1 and 2). A fresh faecal sample was microscopically analysed at this stage, but no abnormalities were detected on direct wet preparation, flotation, sedimentation or on stained smears.
| Table 1. Initial biochemistry results | |||
|---|---|---|---|
| Total protein | 70 | g/L | (33 to 85) |
| Albumin | 33 | g/L | (12 to 41) |
| Urea | 1.5 | mmol/L | |
| Glucose | 13.8 | mmol/L | (9.5 to 25.5) |
| Calcium | 3.03 | mmol/L | (1.77 to 3.66) |
| Ionised calcium | 1.45 | mmol/L | |
| pH reading for ionised calcium | 7.178 | ||
| Phosphate | 1.95 | mmol/L | (0.68 to 5.66) |
| AST | 45 | u/L | (0 to 70) |
| CK | 473 | u/L | (0 to 1,474) |
| Uric acid | 831 | umol/L | (0 to 769) |
| Triglycerides | 2.64 | mmol/L | |
| Sodium | 139.2 | mmol/L | (127 to 159) |
| Potassium | 5.8 | mmol/L | (1.1 to 10) |
| Sodium:potassium ratio | 24 | ||
| Bile acids (starved) | 1 | umol/L | |
| Table 2. Initial haematology results | |||
|---|---|---|---|
| Haemoglobin | 9.3 | g/dl | (5.9 to 9) |
| Haematocrit | 0.29 | l/l | (0.16 to 0.45) |
| Red blood cell | 1.3 | 10^12/L | (0.42 to 1.6) |
| Mean corpuscular volume | 223.1 | Fl | |
| Mean corpuscular haemoglobin concentrate | 32.1 | g/dl | |
| Mean corpuscular haemoglobin | 71.5 | Pg | |
| White blood cells | 3.9 | 10^9/L | (0 to 25) |
| Heterophils | 58% | 10^9/L | (0 to 0.64) |
| Lymphocytes | 20% | 10^9/L | (0 to 16.79) |
| Eosinophils | 0% | 10^9/L | |
| Monocytes | 2% | 10^9/L | |
| Azurophils | 20% | 10^9/L | (0 to 2.29) |
| Basophils | 0% | 10^9/L | (0.02 to 0.92) |
The chameleon was sedated with alfaxalone 10mg/kg IV into his ventral coccygeal vein to allow an accurate full body CT scan, with standard bone and soft tissue algorithms to be performed.
Throughout the CT scan, his optimum temperature range was maintained and monitored using a digital thermometer.
The CT scan demonstrated a suspected right nephrolith, with the right kidney being larger in comparison to the left (Figure 2).
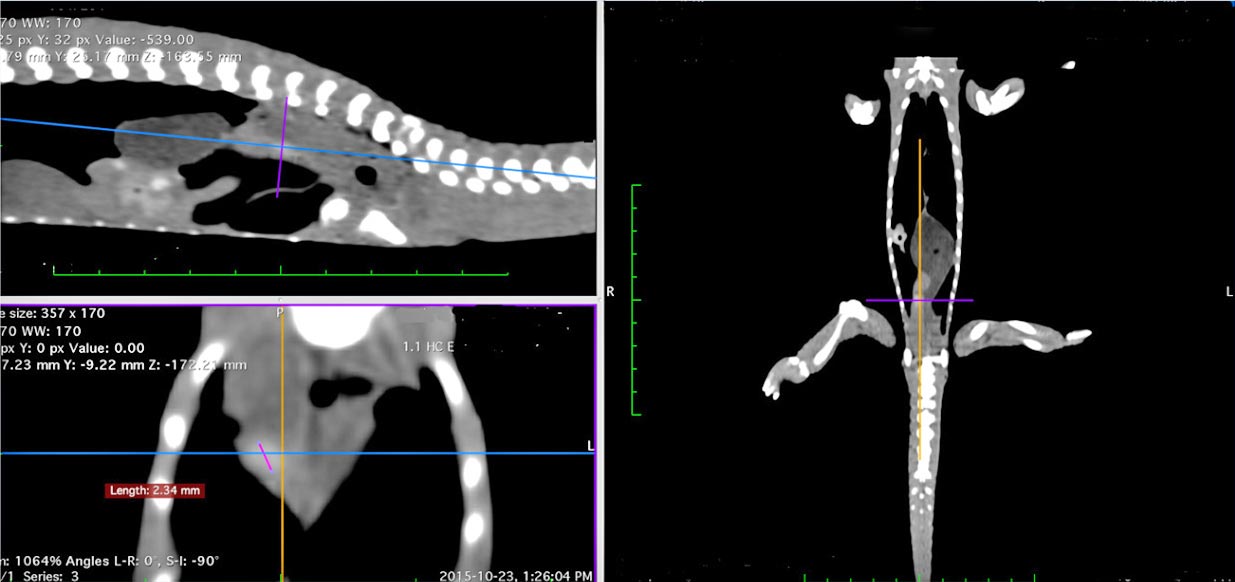
No evidence of visceral gout, pseudo-gout or nutritional bone disease was noted; bone density in general was within normal limits.
Blood results, as demonstrated in Tables 1 and 2, showed a moderate elevation in uric acid, which suggested renal dysfunction. No haematological changes of concern were apparent. It was recommended ultrasound or endoscopic-guided biopsy of the right kidney be performed to allow histopathological analysis, and rule out infective and infiltrative renal disease.
The owner declined further investigation, opting for conservative management. A pharyngostomy tube was placed while the chameleon was still sedated, to allow rehydration and feeding at home. A lateral radiograph (Figure 3) was taken to ensure correct placement of the pharyngostomy tube (Figure 4).
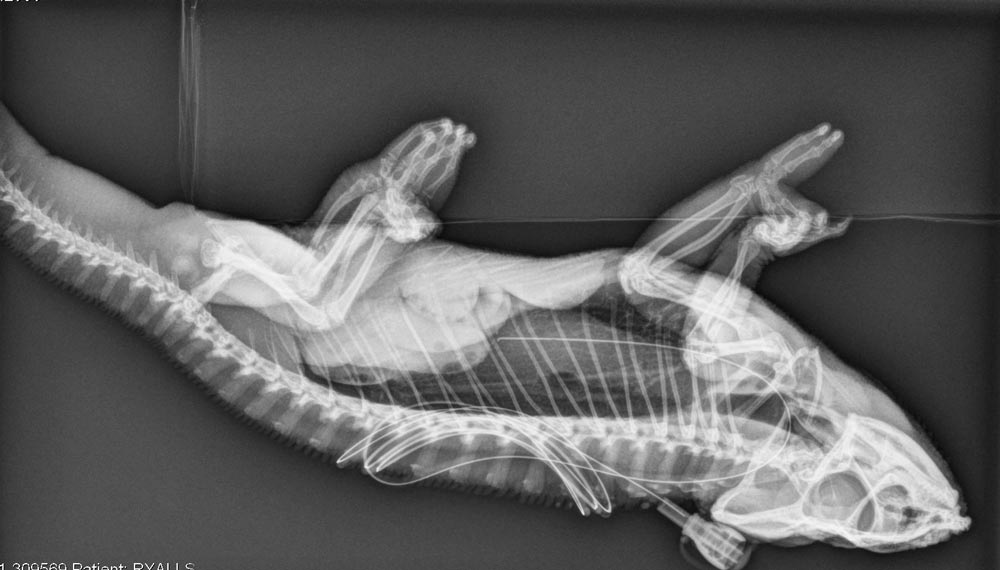

The chameleon was discharged with ceftazidime 20mg/kg, to be given every 72 hours IM for eight consecutive doses. He was to be fed 1% bodyweight of omnivore critical care food twice daily via the pharyngostomy tube, flushed before and after with 1.5ml of tap water.
This percentage was increased over subsequent weeks, eventually reaching 3% bodyweight.
Omnivore critical care food was selected in favour of carnivore critical care food due to its lower content of protein, to avoid further exacerbating the renal disease. A varied diet of gut-loaded live food, dusted with suitable calcium and vitamin supplement, was to be fed daily. It was suggested a new, 12% minimum ultraviolet light should be provided inside the vivarium, spanning the height of the enclosure.
Regular misting and additional water sources, such as a chameleon-specific dripper, were discussed as methods to increase water intake by the chameleon. The ideal temperature ranges and size of enclosure were also discussed in length.
The chameleon was placed in its optimum temperature zone to recover. His recovery was uneventful and he tolerated the pharyngostomy tube well.
Bloods were repeated four weeks later, as shown in Tables 3 and 4, using the same technique previously described. Glucose was slightly raised, but nothing else of concern was noted.
| Table 3. Follow-up biochemistry results | |||
|---|---|---|---|
| Total protein | 59 | g/L | (33 to 85) |
| Albumin | 29 | g/L | (12 to 41) |
| Urea | 1.4 | mmol/L | |
| Glucose | 29.8 | mmol/L | (9.5 to 25.5) |
| Calcium | 2.88 | mmol/L | (1.77 to 3.66) |
| Ionised calcium | 1.56 | mmol/L | |
| pH reading for ionised calcium | 7.217 | ||
| Phosphate | 1.64 | mmol/L | (0.68 to 5.66) |
| AST | 24 | u/L | (0 to 70) |
| CK | 252 | u/L | (0 to 1,474) |
| Uric acid | 433 | umol/L | (0 to 769) |
| Triglycerides | 4 | mmol/L | |
| Sodium | 136.7 | mmol/L | (127 to 159) |
| Potassium | 4.8 | mmol/L | (1.1 to 10) |
| Sodium:potassium ratio | 28 | ||
| Bile acids (starved) | 2 | umol/L | |
| Table 4. Follow-up haematology results | |||
|---|---|---|---|
| Haemoglobin | 8.1 | g/dl | (5.9 to 9) |
| Haematocrit | 0.26 | l/l | (0.16 to 0.45) |
| Red blood cell | 1.04 | 10^12/L | (0.42 to 1.6) |
| Mean corpuscular volume | 250 | Fl | |
| Mean corpuscular haemoglobin concentrate | 31.2 | g/dl | |
| Mean corpuscular haemoglobin | 77.9 | Pg | |
| White blood cells | 6.6 | 10^9/L | (0 to 25) |
| Heterophils | 25% | 10^9/L | (0 to 0.64) |
| Lymphocytes | 56% | 10^9/L | (0 to 16.79) |
| Eosinophils | 0% | 10^9/L | |
| Monocytes | 3% | 10^9/L | |
| Azurophils | 14% | 10^9/L | (0 to 2.29) |
| Basophils | 2% | 10^9/L | (0.02 to 0.92) |
| Thrombocytes | 11 | 10^9/L | |
The owners reported a resolution of swellings in all previously reported areas, a return to normal feeding activity and an increase in his strength of grip; he had stopped falling from branches and was generally more active. A follow-up CT scan to assess the status of the nephrolith was declined.
The pharyngostomy tube was removed and the stoma site left to heal. The chameleon was eventually euthanised when clinical signs returned.
Anorexia in reptiles has various causes, ranging from poor environmental care to numerous infectious and metabolic conditions (Mader, 2006). Investigation to determine the cause is always recommended.
Renal dysfunction is a common occurrence in reptiles, most often caused by prolonged environmental deficiencies (Mader, 2006; Miller, 1998), such as water deprivation or diets too high in protein.
If these environmental deficiencies are prolonged, they can lead to formation of nephroliths (Mader, 2006).
Advanced cases of renal dysfunction are often demonstrated by a serum elevation of phosphorous and uric acid (Miller, 1998). Successful treatment of renal disease in reptiles relies on early detection and swift correction of the underlying causes (Mader, 2006; Miller, 1998; Selleri and Hernandez-Divers, 2006).
This case describes the diagnosis of renal dysfunction, with concurrent presence of a right-sided nephrolith and right-sided renomegaly, likely caused by dehydration, poor ultraviolet provision and a diet too high in protein in a panther chameleon.
Diffuse renal infiltrates, such as infection or neoplasia, could not be ruled out as further investigations were declined by the owner.
However, in this case, correcting the chameleon’s environmental conditions, including providing a more varied and better supplemented diet, as well as increasinghis fluid intake and supportive feeding, appeared to reverse the renal dysfunction and allowed the subsequent symptoms to resolve.