9 Dec 2020
Elisabetta Mancinelli describes the clinical presentation and diagnosis of this condition, as well as management and surgical options.
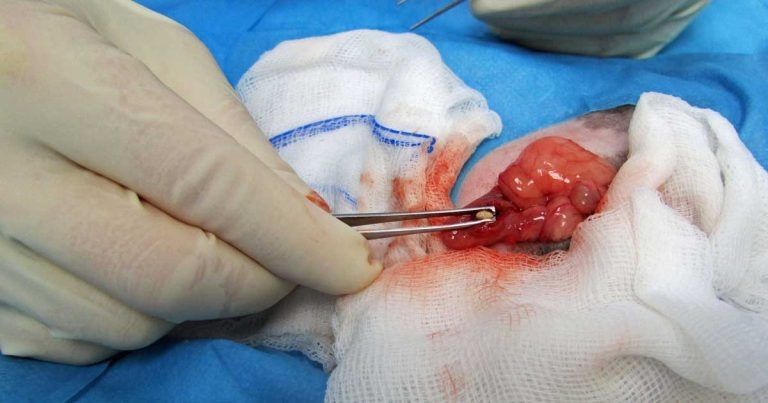
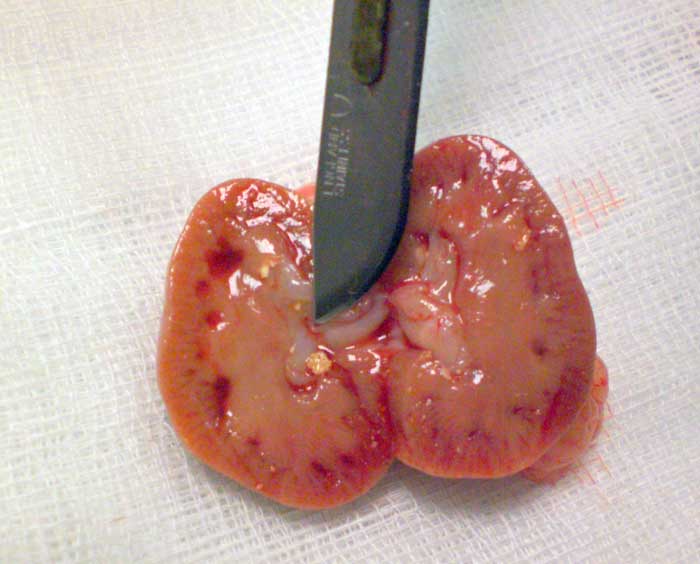
Figure 1. Stones can be localised anywhere in the urinary tract of guinea pigs. In this case, a stone was removed from a guinea pig’s bladder.
The order Rodentia is the largest mammalian order, including more than 1,700 species (about 40% of all mammalian species) distributed worldwide. These animals differ widely in size, behaviour, feeding habits, anatomy and physiology.
Based on anatomical differences of their jaw muscles or “zygomasseteric system” (the anatomical arrangement of the masseter muscle of the jaw and the zygomatic arch of the skull), rodents are grouped into three suborders:
Guinea pigs are hystricomorph rodents and are among the most popular pets within the UK.
Many people consider guinea pigs “disposable” pets because they can be purchased for a low price and are supposedly easy to care for, meaning a trip to the vet does not represent value for money. Nevertheless, many owners do recognise the “special needs” of this species and seek appropriate veterinary care for them.
Many clients often show a strong emotional attachment towards guinea pigs and are becoming increasingly demanding, expecting knowledge and exceptional quality care for these small rodents.
Consideration needs to be given to the fact guinea pigs are prey species, possibly even more adept than rabbits at disguising signs of illness – leading many owners to conclude that when they do expire, they do so suddenly and for no apparent reason. However, when veterinary intervention is sought, it is usually not until the animal is in a critical – or even moribund – state.
Inappropriate diet and husbandry are often responsible for many of the common disorders seen in pet guinea pigs (Jenkins, 2010). Dental disease (36.3%), skin disorders (33.3%), urolithiasis and ovarian cystic disease are among the most common conditions reported in guinea pigs (Minarikova et al, 2015). However, a challenging mismatch seems to exist between the conditions considered as common by the veterinary profession, and those found in the literature (Robinson et al, 2017).
Urolithiasis is commonly reported and mostly affects middle-aged to older guinea pigs (older than two-and-a-half years of age), although it can also be seen in younger animals.
Stones may be found anywhere along the urinary tract (from the bladder, urethra, ureter and kidneys) and sometimes in the seminal vesicles of males (Hoefer and Latney, 2009; Hawkins and Bishop, 2012; Figures 1 to 4).
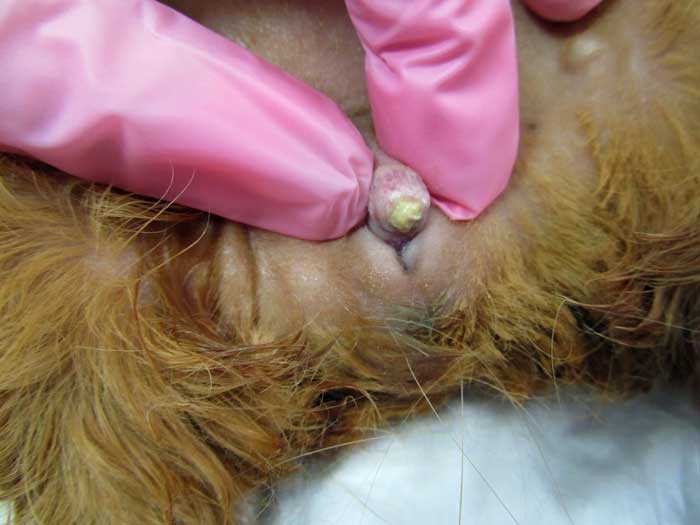
A case of urethral diverticulum with an intraluminal urolith has also been reported in a five-year-old sexually intact female guinea pig evaluated because of mild dysuria and a SC mass located cranioventral to the urogenital openings (Parkinson et al, 2017).
A study found an equal distribution of urinary calculi between males and females older than two years of age. Those located in the ureters seemed to predominate in males (Hawkins et al, 2009).
Female guinea pigs seem to be more prone to bacterial cystitis due to the close anatomical association of the urethral orifice with the anus, which allows for contamination with faecal bacteria such as Escherichia coli (Peng et al, 1990).
Some authors consider Peruvian guinea pigs to have a predisposition to develop urolithiasis, but this has not been confirmed (Hawkins et al, 2009). Many reports of this condition exist in the literature (Spink, 1978; Stuppy et al, 1979; Okewole et al, 1991; Fehr and Rappold, 1997; Gaschen et al, 1998; Stieger et al, 2003; Eshar et al, 2013; Parkinson et al, 2017).
Its aetiopathogenesis remains unclear, although it has been suggested the alkaline pH and high mineral content of normal guinea pig urine may favour crystal formation and precipitation (Hawkins and Bishop, 2012).
Risk factors contributing to urolithiasis may also include inadequate water intake, urine retention, inadequate cage hygiene, obesity, lack of exercise, food items with high mineral content (such as calcium and oxalate), renal disease, and excessive vitamin or mineral supplementation (Hawkins, 2011).
Diet seems to play a major role, mainly pointing at calcium and oxalate as main risk factors in stone formation. In a study that looked into risk factors associated with development of urolithiasis, affected guinea pigs were more likely to be fed a diet high in overall percentage of pellets, low in percentage of hay, and with a lesser variety of vegetables and fruits (Hawkins et al, 2008).
Calcium-containing stones – such as calcium oxalate (historically) and calcium carbonate – are, in fact, most commonly reported. Available data seem to suggest calcium carbonate calculi are overwhelmingly prevalent (88% to 93%; Hawkins et al, 2008; 2009; Osborne et al, 2009).
Infections and mechanical factors may also predispose to stone formation.
Symptoms generally depend on size and location of the stone/s.
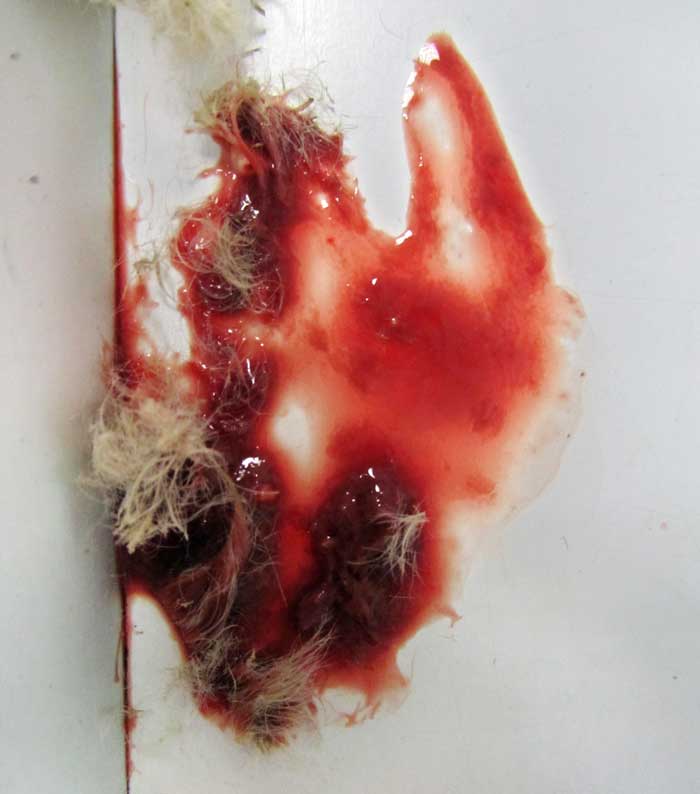
Haematuria (Figure 5), stranguria and dysuria may be seen with cystic or urethral calculi. More commonly, clinical signs may reflect abdominal pain – such as a hunched posture (Figure 6) and teeth grinding – or non-specific signs of disease such as anorexia, lethargy and weight loss. These symptoms may be seen more frequently when calculi are higher in the urinary tract.

Although presentation may be acute, it is common for signs and symptoms to progress over days to weeks in affected rodents.
On physical examinations, patients are usually painful on palpation of the caudal abdomen and may reveal an exaggerated response when the bladder is palpated.
It should be kept in mind most normal guinea pigs may resist abdominal palpation, and routinely struggle and vocalise.
In some cases, blood may be visualised from the urethral orifice. This should be differentiated from blood emerging from the vaginal orifice, which is most commonly seen with disease of the female reproductive tract.
A detailed history, including dietary history, is extremely important in association with a complete physical examination.
Urolithiasis should be suspected in any rodent with changes in its urinary output or with non-specific signs of illness. A blood and urine sample should be collected for complete haematobiochemistry and urinalysis to rule out kidney involvement and the presence of infection. Laboratory findings may reflect a high calcium diet (for example, hypercalcaemia) or may provide evidence of infection.
Urine from healthy guinea pigs can undergo marked physiological visual changes in turbidity and colour multiple times within a period of 72 hours. This variability is reported as dependable on feeding, porphyria and physiological crystalluria (Rueloekke et al, 2016a).
The median value for urinary protein to creatinine ratio (UPC) was found to be 1.60 (range 0.00 to 15.90). No sex differences (p=0.11) were found and no correlation was seen between age and UPC (r2=0.00 and p=0.76; Rueloekke et al, 2016b).
This study revealed the UPC ratio in apparently healthy guinea pigs may be much higher than values from healthy dogs and cats. These findings indicated that either subclinical renal disease is a frequent condition in guinea pigs or physiological proteinuria of non-renal origin is a common finding in voided guinea pig urine. Therefore, UPC seems inapplicable as a diagnostic parameter for suspected renal disease.
In another study, the mean specific gravity of 44 guinea pigs with urinary calculi was found to be 1.015 +/- 0.008 (range 1.004 to 1.046) and the urine pH was 8.4 +/- 0.5 (range 6.5 to higher than 9; Hawkins et al, 2009). Haematuria, mucus and lipid droplets were seen on sediment examination in the same study. Crystals type more commonly seen on sediment examination were calcium carbonate, oxalate and struvite. However, as in other species, this may not be predictive of the urinary calculi composition (Hawkins et al, 2009).
Culture of the urine collected in a sterile way (prior to antibiotic use) or of the bladder wall can direct the antibiotic choice when the sediment examination reveals the presence of bacteria, red and/or white blood cells. However, bacterial cultures of urine, bladder wall specimens and calculi were mostly negative in a 2009 study (Hawkins et al, 2009).
It must be stressed the majority of the pigs considered in that study were receiving antimicrobials. In those pigs with positive culture, Corynebacterium renale was the most commonly identified bacteria. Facklamia species, Streptococcus bovis (equinus group), Enterococcus species, E coli, Streptococcus viridans, Proteus mirabilis and Staphylococcus species were also identified.
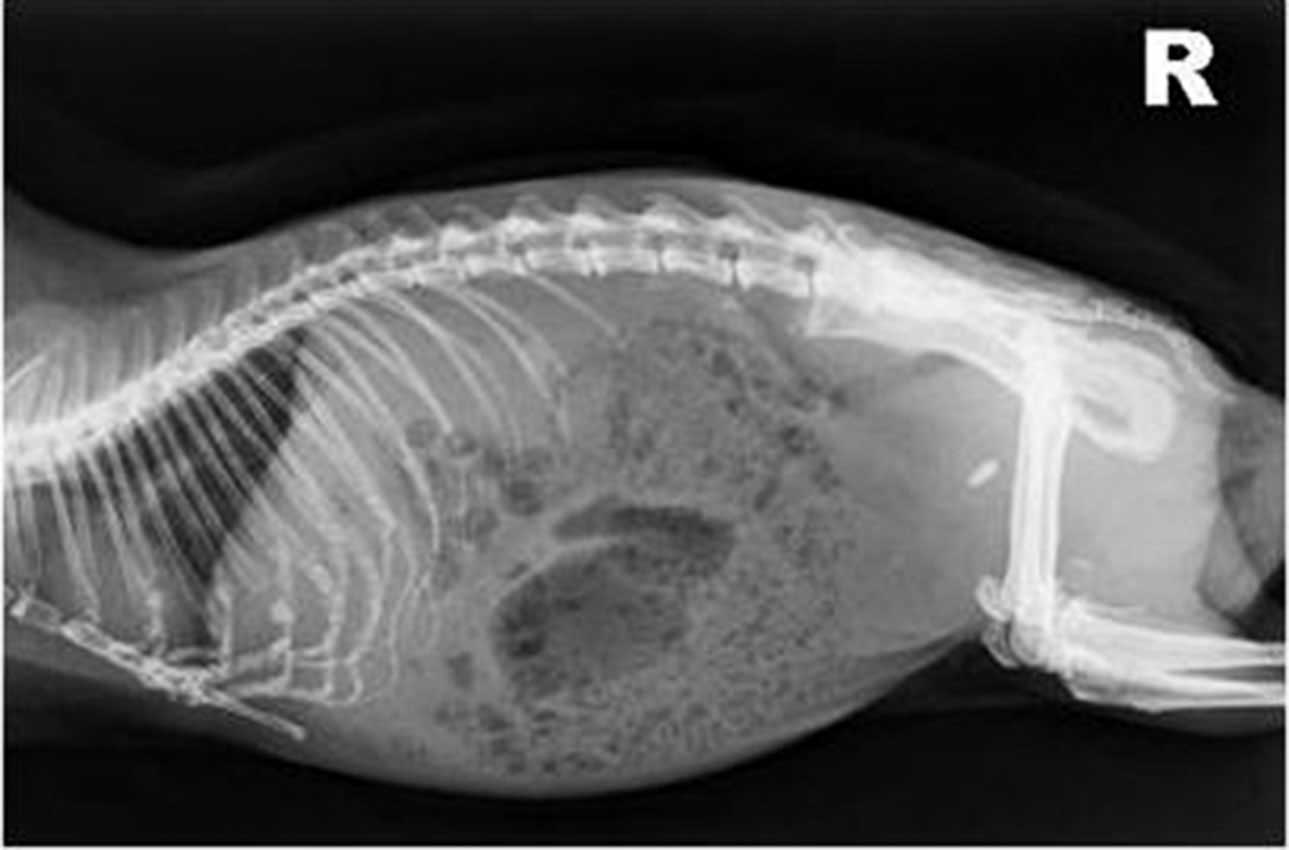
Abdominal radiographs are also valuable as urinary calculi in guinea pigs are generally radiopaque (Figure 7).
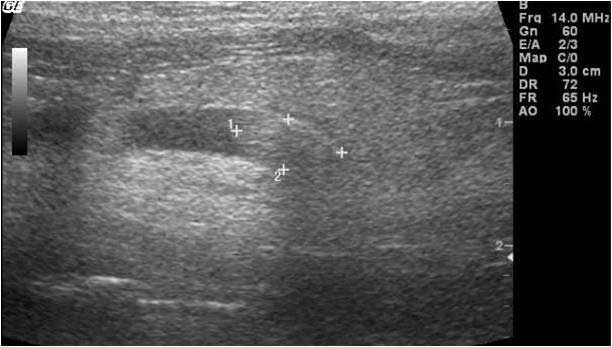
Abdominal ultrasound can help to confirm a presumptive diagnosis made using radiographs, or it may be necessary to further localise stones in the urethra, ureter or kidneys. Ultrasonography also allows evaluation of changes within kidneys, ureters or bladder (for example, hydronephrosis, hydroureter or mucosal inflammation; Figure 8), as well as other abdominal (non-urinary) pathologies. Large amounts of gas or ingesta within the gastrointestinal tract, a common occurrence in guinea pigs, may impede visualisation.
When bladder calculi and hypercalciuria are difficult to differentiate radiographically, ultrasonography of the bladder may reveal an acoustic shadow if a calculus is present, whereas points of echogenic reflections – a “snowstorm” effect – will be seen if crystals only are present (Keeble and Benato, 2013).
Contrast cystography and urethrography may provide more specific information regarding the bladder and urethra. An IV pyelogram – or excretory urography – may be used to evaluate the size, shape, position and internal structure of the kidneys, ureter and urinary bladder, and is especially helpful at assessing the upper urinary tract (kidneys and ureters) for calculi, masses or obstructive lesions (Fisher, 2015).
More recently, ultrasound-guided percutaneous antegrade pyelography has been described as a useful and easy-to-perform diagnostic technique in guinea pigs with hydronephrosis and hydroureter (D’Ovidio et al, 2020).
Further to this, detailed images can be obtained with a CT scan and contrast agents can be used to elucidate functional abnormalities.
Mineral evaluation of the calculi removed can be performed, taking care that the lab chosen for such analysis is able to use techniques capable of differentiating between calcium carbonate and calcium oxalate (Hawkins and Bishop, 2012).
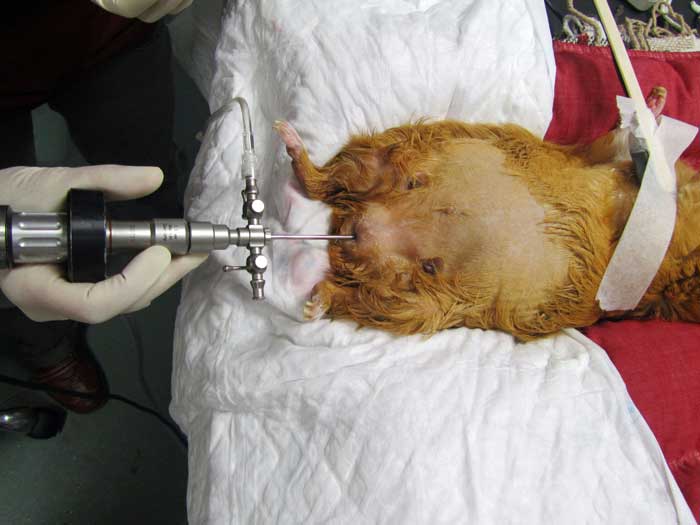
Cystoscopy may also be a useful diagnostic tool for direct visualisation of the mucosal surface of the urethra and urinary bladder (Figure 9), and allows obtaining biopsies of mucosal lesions (Wenger and Hatt, 2015).
Because the exact mechanism of stone formation is not known in guinea pigs, clear directions for prevention and dissolution of calculi cannot be given in this species.
Surgical removal of the urinary calculi is considered the treatment of choice, as medical treatment of urolithiasis is often unrewarding and ineffective (Hawkins and Bishop, 2012).
Recurrence of the condition once the calculi have been surgically removed is high; owners should be advised and informed accordingly.
Medical management may include a combination of the following options.
Water intake should be increased, and dietary calcium and oxalate intake reduced.
According to one study, dietary modification aimed at including more hay (avoiding alfalfa hay and alfalfa-based pellets), and a wider variety of fruits and vegetables seemed to decrease the risk of urolith development in pet guinea pigs (Hawkins, 2008).
Dietary calcium and oxalate levels should be reduced, but not eliminated. Switching to grass, timothy or oat hay while feeding a lower overall percentage of pellets may be of help because of the lower calcium level and higher fibre content of the diet.
Food containing high levels of oxalate – such as spinach, kale, celery, parsley and strawberries – should be limited, especially in conjunction with a low-calcium diet. Mineral blocks or other mineral supplements should be removed from the diet.
It is important to remember severe dietary calcium restriction can be dangerous in guinea pigs and cases of metabolic bone disease (for example, fibrous osteodystrophy) have been reported following drastic dietary measures for prevention of urinary calculi (Hawkins and Bishop, 2012).
Vitamin C is extremely important in guinea pigs, as they cannot synthesise their own.
When supplementation is limited to 25mg/kg to 100mg/kg per day, it is unlikely to result in excess oxalate levels (vitamin C has high oxalate levels). Fresh cabbage, kale and oranges are good natural sources of vitamin C.
Vitamin C can also be added to drinking water (200mg/L to 400mg/L daily), but the vitamin is unstable and inactivated quickly with light. Tablets can also be crushed and sprinkled over vegetables and fruit (Hoefer and Latney, 2009).
Potassium citrate is often used in human medicine to help prevent the formation of calcium-based stones (Finkielstein and Goldfarb, 2006).
Citrate in the urine binds to calcium, reducing ion activity, alkalinising the urine and inhibiting crystal formation (Hoefer and Latney, 2009; Hawkins and Bishop, 2012).
Dissolution therapy – as used in animals with acidic urine to inhibit calcium stone crystallisation in the urine by using agents such as potassium citrate – is controversial in a species with alkaline urine. However, some clinicians find the use of urinary acidifiers – such as potassium citrate – helpful in reducing stone formation after surgical removal (Hoefer and Latney, 2009).
The reported dose in guinea pigs is 30mg/kg to 75mg/kg orally twice daily (Hawkins and Bishop, 2012). However, other sources also suggest 150mg/kg twice daily (Hoefer and Latney, 2009).
Thiazide diuretics, such as hydrochlorothiazide (HCTZ), act by reducing reabsorption of electrolytes in the distal tubules, therefore increasing excretion of water and electrolytes (Silverstein and Hopper, 2015).
They are also known as calcium-sparing diuretics as they have been shown to reduce levels of urinary calcium and are frequently used in human medicine to reduce recurrence of calcium-based stones (Finkielstein and Goldfarb, 2006).
HCTZ is sometimes used alongside potassium citrate, as the latter works to prevent potential hypokalaemia that may be seen with thiazide diuretics, as well as the additional aforementioned properties (Odvina et al, 2003; Pak, 2008).
It is important to ensure multiple water sources are available, to prevent dehydration. Its use is contraindicated when renal impairment is suspected.
Pentosan polysulphate is used in cats with idiopathic cystitis. Based on this, it has been suggested this drug may be beneficial in the management of chronic idiopathic, non‑obstructive lower urinary tract disease in guinea pigs. However, currently no evidence exists to support this claim (Hedley, 2020).
Supportive and symptomatic treatments should be integrated in the treatment plan, and include appropriate rehydration, adequate pain control, assisted feeding, antibiotic treatment (when indicated) and following appropriate testing.
Culture and sensitivity should always be considered as they can be extremely helpful for a more specific medical therapy.
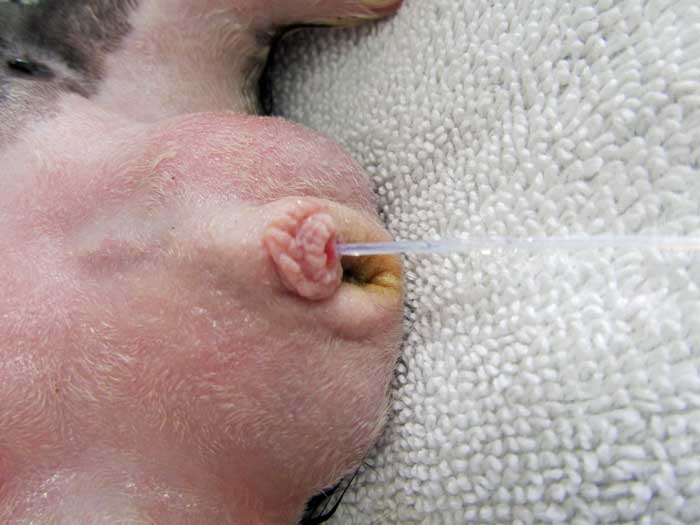
The female guinea pig may be catheterised to flush the bladder under anaesthesia and attempt removal of smaller calculi, but the reduced size of the male urethra makes this procedure more dangerous (Hawkins and Bishop, 2012).
However, catheterisation of the male guinea pig can be performed – taking care to avoid trauma to the urethra (Figure 10) – to allow retrograde flushing of the bladder, which may help relieve urethral obstructions (Brown and Mans, 2007).
Cystoscopic urolith removal may also be a more rapid and less invasive alternative to surgical removal when the calculi are sufficiently small (Pizzi, 2009; Wenger and Hatt, 2015). Stone fragmentation and removal by cystoscopic-guided transurethral laser lithotripsy has also been reported (Coutant et al, 2019)
If non-invasive options are not feasible and the guinea pig is not obstructed, supportive care – including fluids and assisted feeding – should be provided and surgery for removal of the stone scheduled appropriately, depending on the clinical conditions of the patient.
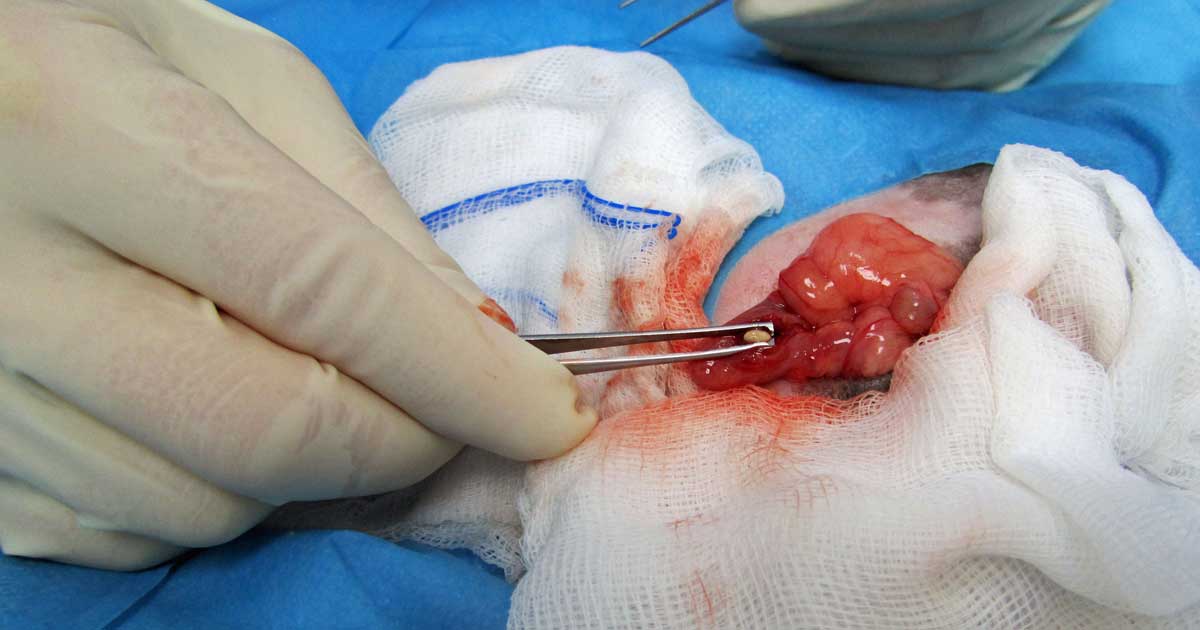
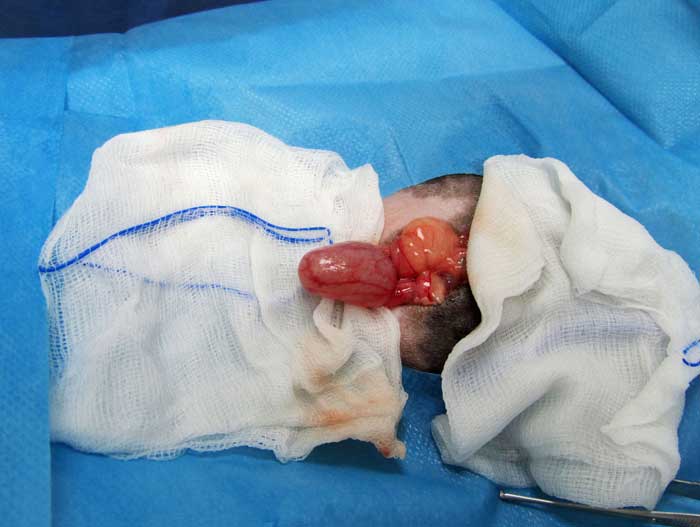
Cystotomy is performed as routine (Hoefer and Latney, 2009; Bennett, 2012; Figure 11). An urethrotomy may be performed to retrieve urethral uroliths (Figure 12) and staged procedures may be considered for removal of uroliths from two different locations within the guinea pig urinary tract (Fawcett and Roussille, 2016).
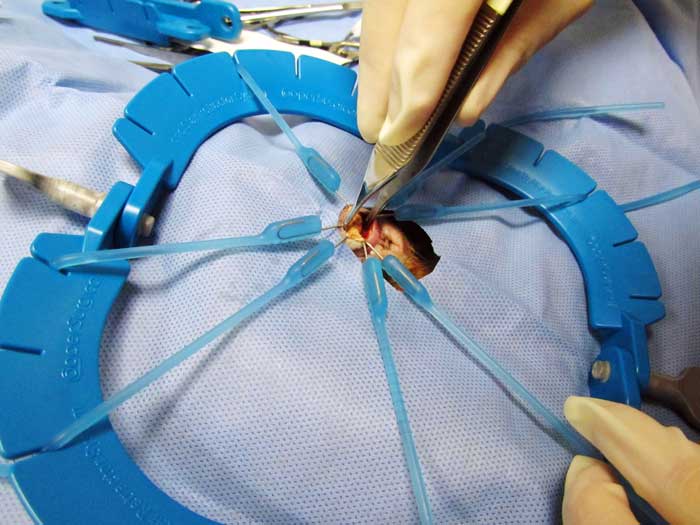
Surgery is also considered the preferred procedure for relief of ureteral obstructions. Ureteral stones should be milked down into the bladder, if possible.
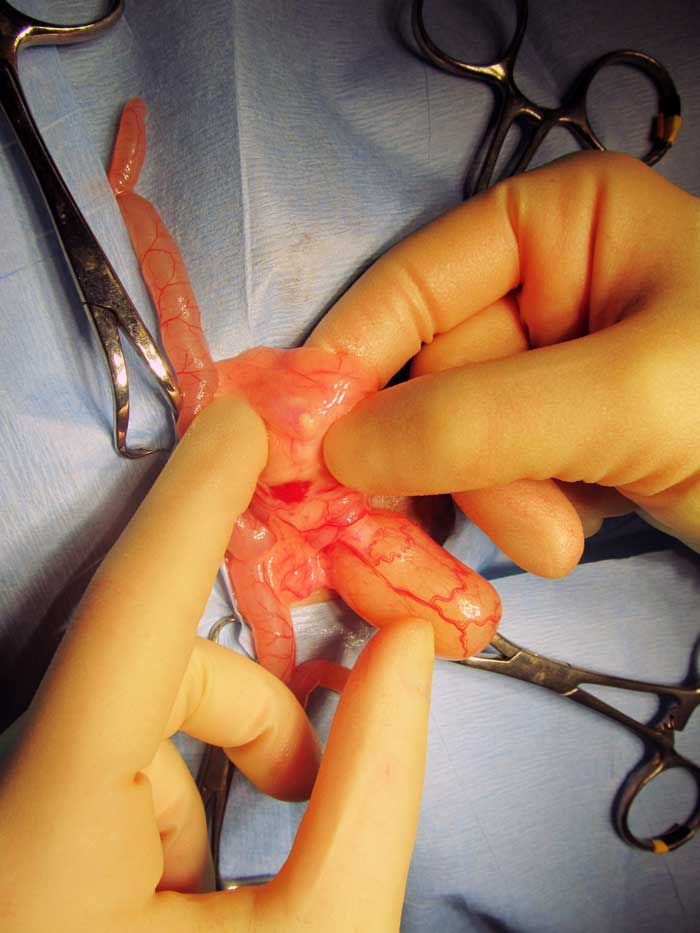
Ureterotomy has been described in guinea pigs (Gaschen et al, 1998; Stieger et al, 2003; Bennett, 2012; Mancinelli, 2012; Figure 13). However, significant complications following this procedure are reported in dogs and cats – including leakage and ureteral stricture – and they may be expected in guinea pigs as well, especially given the challenges of their relatively smaller size. In cats and dogs, ureterotomy can be avoided with placement of ureteral stents, allowing bypassing the obstructed section of the ureter. This procedure has not been reported in guinea pigs.
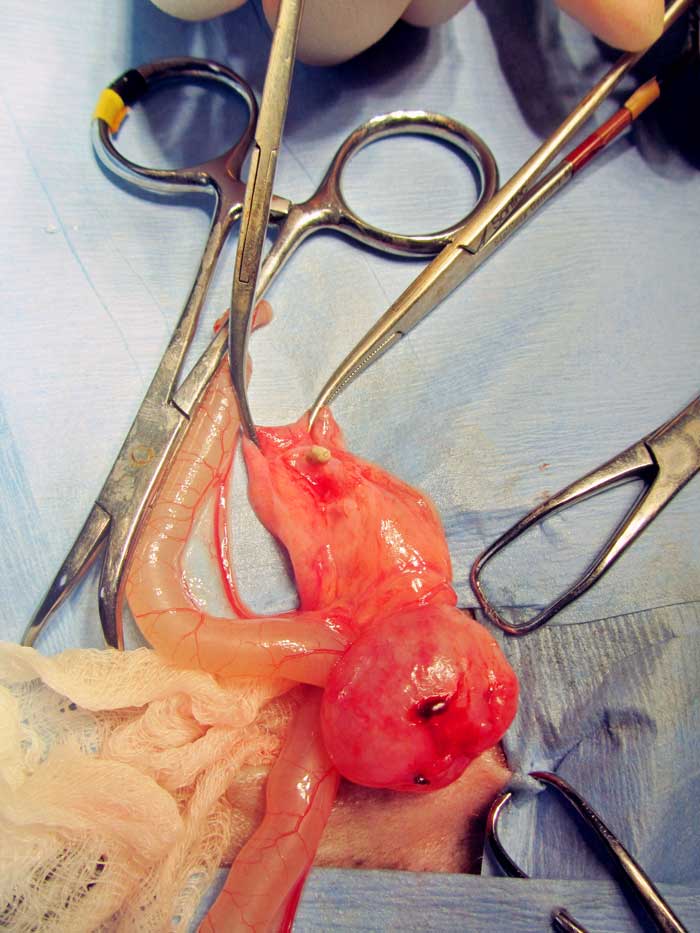
The palliative clinical management of a complicated ureteral obstruction in a guinea pig has been described using an ultrasound-guided percutaneous antegrade nephrocentesis and hydropropulsion (Eshar et al, 2013).
Urolithiasis commonly affects middle-aged to older guinea pigs. However, its pathogenesis remains largely unknown. Further research is, therefore, warranted into the aetiology of this condition that may provide guidelines for prevention of urolith formation in this species.
Urolithiasis should be considered a differential in any sick guinea pig. Owners should also be encouraged to inspect the ventral region of guinea pigs regularly to detect signs of urogenital disease.