24 Nov 2021
Blood collection options in rodents
Elisabetta Mancinelli describes methods and locations to obtain blood, and volumes considered safe to withdraw in these patients.
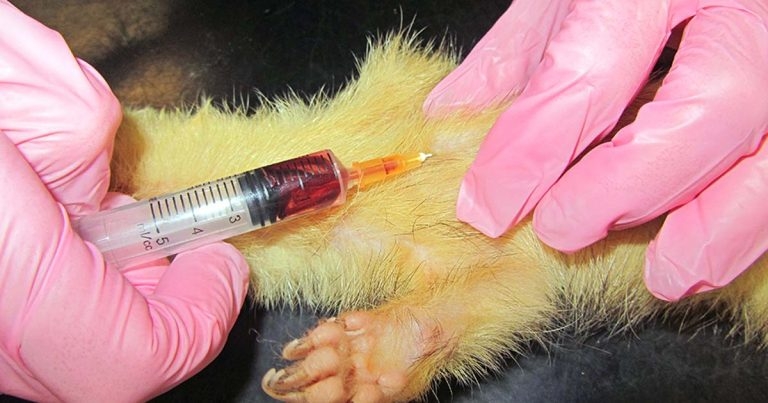
Figure 8. The cranial vena cava is routinely used in ferrets.
- This is an extract from an article “Blood collection options in exotic small mammals”, originally published in Vet Times 46.45 (14 November 2016).
A blood sample is often necessary in many exotic species for general health checking, including routine haematology and biochemistry and/or for measurement of specific parameters.
The method of blood collection depends on the amount required, frequency of sampling, parameters to be measured and skills in obtaining the sample (Campbell, 2012). Fasting before blood collection in small pet mammals is usually not required unless specific tests are requested – for example, blood glucose in a ferret with suspected insulinoma.
The maximum amount of blood that can be safely taken in a single draw (assuming it is healthy) is equal to 1% of the animal’s bodyweight (BW) or 10% of the animal’s total blood volume. If more frequent sampling is required (for example, more than every two weeks), 0.5% of the BW may need to be withdrawn at any one time as it may take two weeks or longer for all the blood constituent to return to normal, although the blood volume will be restored within 48 hours from collection.
It may take even longer in unhealthy patients (Campbell, 2012). However, consideration should be given to the fact differences may be seen in total blood volume when species and age of the animal are compared.
It is, therefore, best to err on the side of caution by using a lower blood sampling volume estimate (0.8%) and collecting that volume no more than once every 14 days, or follow published charts that compare the sample volume that can be collected when limited to 0.08%, 1% or 10% of the animal’s BW (Joslin, 2009).
The Mongolian gerbil has a rapid turnover rate for erythrocytes, which have an average 10-day lifespan. Therefore, the response to blood loss from haemorrhage is much faster in this species compared with other small exotic mammals, and blood samples can be collected more frequently without adverse physiologic effects in most cases (Joslin, 2009).
In small mammals, blood can be placed in ethylenediamine tetra-acetic acid (EDTA) and/or lithium heparin for haematology and biochemistry, as the sample is often small.
Rodents
In rodents, it is advisable to collect blood only from stable patients and with a clear idea of the maximum blood volume that can be safely withdrawn from a healthy or sick animal (Lennox and Bauck, 2012).
Blood collection studies in rats have shown removal of 7.5% of the total blood volume (7.2 +/- 1.19ml/100g BW, which, in an average 300g rat, would correspond to 21.6 ml) over 24 hours are not likely to have any biological effect with complete recovery in 48 hours (Nahas and Provost, 2002). If this amount was to be collected from a 300g rat, it would correspond to 1.62ml. If 1% of the BW was to be collected from the same animal, the blood volume collected would correspond to 3ml.
However, blood can be collected in healthy rats up to 20% of the total blood volume over 24 hours (4.32ml in a 300g rat) without causing any ill effects or compromising the animals’ welfare (Campbell, 2012).
In clinical settings, large samples are not often required; therefore, a single blood draw is deemed safe following the general 1% BW rule. General anaesthesia may be required in many rodents for blood sampling, as the techniques used for restraint in laboratory rodents are often not appropriate for pet animals; however, this may have an effect on test results. In rats, following exposure to 4% isoflurane for five minutes caused a slight downward trend in the red blood cell parameters and in potassium, and an increase in glucose. These changes probably represent an effect of prolonged exposure to isoflurane. Therefore, to avoid variation in these parameters, the duration of exposure should be limited to three minutes (Nahas and Provost, 2002).
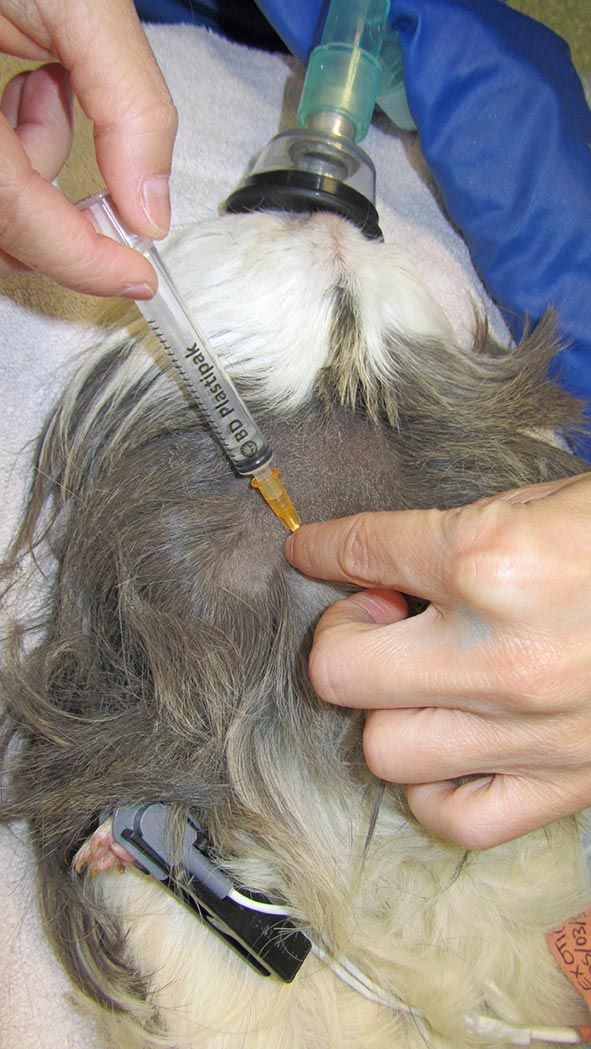
Short sedation may be a valid alternative. The cranial vena cava is the largest easily accessible vessel in rodent species (Lennox and Bauck, 2012; Quesenberry, Donnelly and Mans, 2012), but may result in pericardial sac penetration and bleeding into the thoracic cavity if an inappropriate technique is used (Lichtenberger and Hawkins, 2009). The injection site is just cranial to the first rib, 0.3cm to 0.8cm lateral to the manubrium when the animal is in dorsal recumbency. The needle, attached to a syringe, is inserted at a 30° to 45° angle in the direction of the opposite femoral head (Jekl et al, 2005; Lichtenberger and Hawkins, 2009). In species with an underdeveloped clavicle, such as in guinea pigs and chinchillas, the needle is inserted cranial to the manubrium and first rib (Figures 1 and 2).
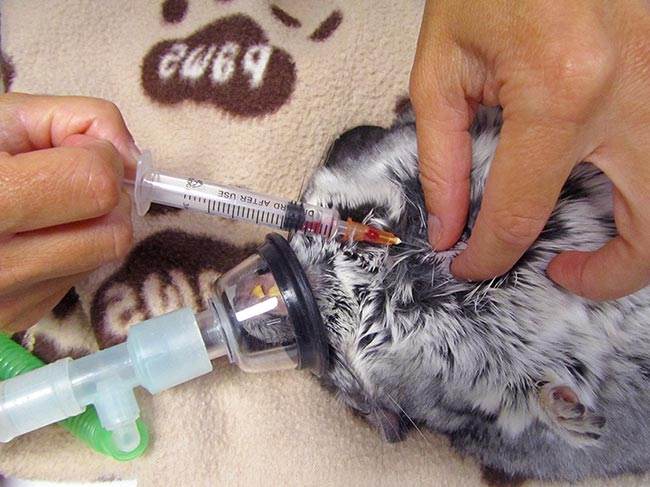
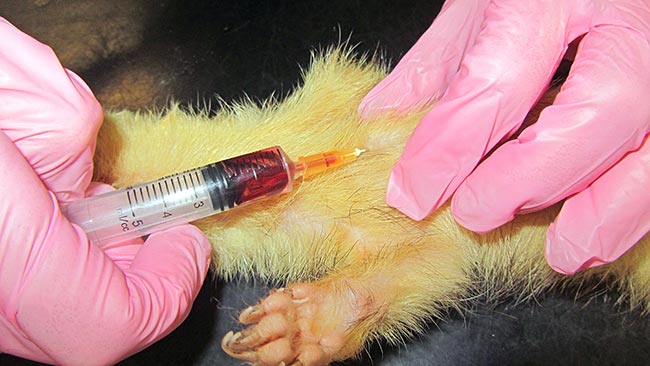
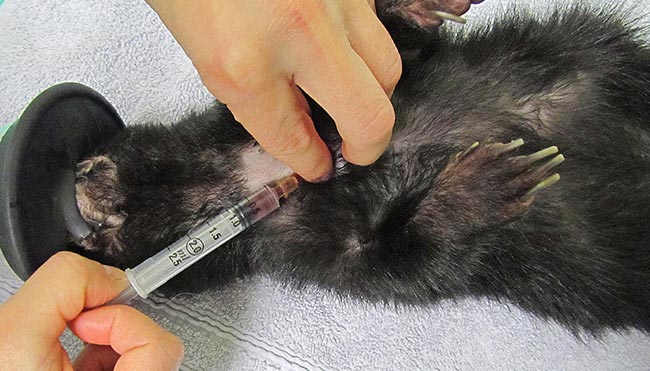
In rodents with a developed clavicle (rat, hamster, mouse and gerbil), the needle needs to be inserted between the clavicle and the first rib (Lichtenberger and Hawkins, 2009; Figures 3 to 5).
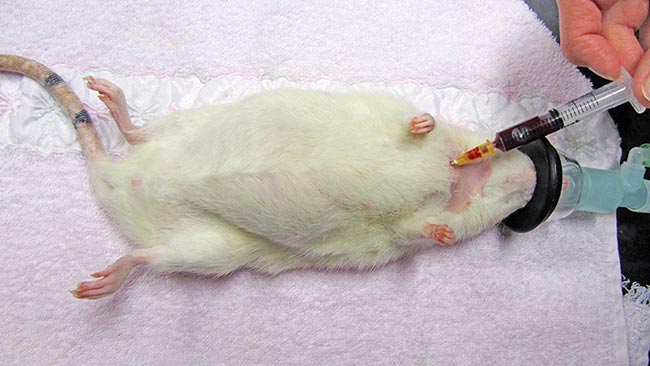
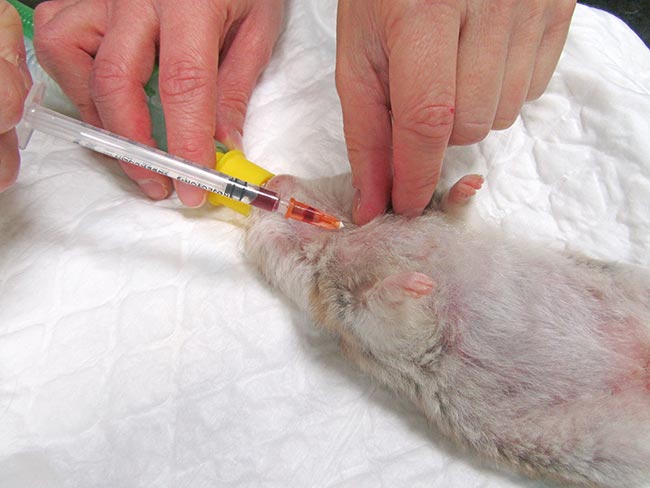
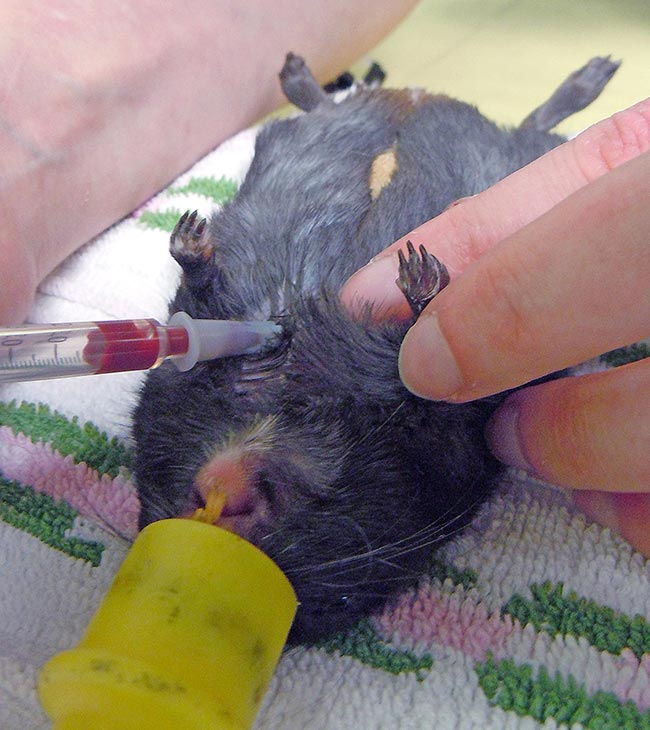
Jugular venipuncture can be challenging in species that have short, thick necks with large fat coverage – making the veins difficult to locate, especially in guinea pigs. Restraint may be stressful and anaesthesia or sedation are usually required (Lichtenberger and Hawkins, 2009). The lateral saphenous and the cephalic vein may be used, but can be difficult to locate, collapse easily and may yield only minimal blood volumes.
The femoral vein may be used in anaesthetised rodents. However, it is advised pressure is held for several minutes after collection in case the femoral artery is inadvertently sampled (Lichtenberger and Hawkins, 2009).
The lateral tail vein (on either side) can be used in rats, mice and gerbils; also in rats, the ventral tail artery may be used. The artery is located slightly off the ventral midline. An appropriately sized needle can be inserted one-third of the length of the tail from its base at a 30° angle. Warming the tail to allow vasodilation may help to achieve easier access. The pressure of the artery fills the syringe with blood, thus adequate pressure is required after sampling. The lateral saphenous vein and femoral vein may also be used in small mammals. Other collection sites used in laboratory settings are not recommended for pets.
Ferrets and skunks
Taking blood from a ferret is relatively easy and may be done without the need for general anaesthesia. Most ferrets can be distracted with food items or multivitamins. Avoid products containing sugars as this may affect the blood glucose levels. Fasting samples may be required for glucose determination. Two sites are commonly used for venipuncture – the cranial vena cava and the jugular vein.
The cranial vena cava can be accessed by restraining the ferret in dorsal recumbency with its head and neck extended and forelimb along the side. An appropriately sized needle is inserted between the manubrium of the sternum and the first rib at a 45° angle to the body of the animal, and pointing towards the opposite hindlimb (Figure 6).
The actual site of venipuncture is either the right or left brachiocephalic trunk or the anterior vena cava, depending on the point of entry and the depth of needle penetration (Quesenberry and Orcutt, 2012). There is no risk of cardiac puncture as the heart is located caudally in the thoracic cavity.
The jugular vein is usually more superficial and lateral than in dogs and cats, and runs between the thoracic inlet and the angle of the mandible when the head and neck are extended (Campbell, 2012). The technique is similar to that used in the cat and, when smaller samples are needed, the cephalic or saphenous veins can be used.
Use of the ventral tail artery has been described, but is considered painful and requires anaesthesia (Bleakley, 1980). Marini et al (1994) studied the effect of isoflurane on a ferret’s haematologic variables and found they all decreased – starting at the beginning of induction and reaching the maximal effect at 15 minutes. Although these alterations appear to be well-tolerated in healthy ferrets, care should be exercised when anaemic, geriatric or debilitated ferrets are anaesthetised with isoflurane. Similar techniques for blood sampling may be used in skunks (Figure 7).
References
- Bleakley SP (1980). Simple technique for bleeding ferrets (Mustela putorius furo), Lab Anim 14(1): 59-60.
- Campbell TW (2012). Mammalian hematology: laboratory animals and miscellaneous species. In Thrall MA, Weiser G, Allison RW and Campbell TW (eds), Veterinary Hematology and Clinical Chemistry (2nd edn), John Wiley and Sons, Iowa: 225-237.
- Jekl V, Hauptman K, Jeklova E and Knotek Z (2005). Blood sampling from the cranial vena cava in the Norway rat (Rattus norvegicus), Lab Anim 39(2): 236-239.
- Joslin JO (2009). Blood collection techniques in exotic small mammals, J Exotic Pet Med 18(2): 117-139.
- Lennox AM and Bauck L (2012). Basic anatomy, physiology, husbandry and clinical techniques. In Quesenberry KE and Carpenter JW (eds), Ferrets, Rabbits and Rodents Clinical Medicine and Surgery, Elsevier Saunders, St Louis, Missouri: 339-353.
- Lichtenberger M and Hawkins MG (2009). Rodents: physical examination and emergency care, BSAVA Manual of Rodents and Ferrets, Gloucester: 18-31.
- Marini RP, Jackson LR, Esteves MI et al (1994). Effect of isoflurane on hematologic variables in ferrets, Am J Vet Res 55(10): 1,479-1,483.
- Nahas K and Provost JP (2002). Blood sampling in the rat: current practices and limitations, Comp Clin Path 11(1): 14-37.
- Quesenberry KE, Donnelly TM and Mans C (2012). Biology, husbandry, and clinical techniques of guinea pigs and chinchillas. In Quesenberry KE and Carpenter JW (eds), Ferrets, Rabbits and Rodents Clinical Medicine and Surgery, Elsevier Saunders, St Louis, Missouri: 279-294.
- Quesenberry KE and Orcutt C (2012). Basic approach to veterinary care. In Quesenberry KE and Carpenter JW (eds), Ferrets, Rabbits and Rodents Clinical Medicine and Surgery, Elsevier Saunders, St Louis, Missouri: 13-26.
