20 Jul 2021
Common conditions of guinea pigs – part 1
Elisabetta Mancinelli begins this series by discussing urinary tract and gastrointestinal disorders that are frequently seen in these patients.

Rodentia is the largest of mammalian orders, and includes more than 1,700 species (about 40% of all mammal species) distributed worldwide. These animals differ widely in size, behaviour, feeding habits, anatomy and physiology.
Based on anatomical differences of their jaw muscles, rodents are grouped into three suborders, namely:
- the Myomorpha (“rat-like” or “mouse-like”)
- the Sciuromorpha (“squirrel-like”)
- the Hystrichomorpha or Caviomorpha (“porcupine-like” or “guinea pig-like”).
Guinea pigs are rodents belonging to the suborder Hystrichomorpha and are among the most popular pets kept in the UK.
Inappropriate diet and husbandry are often responsible for many of the common disorders seen in pet guinea pigs (Jenkins, 2010). Dental disease (36.3%), skin disorders (33.3%) and ovarian cystic disease are among the most common conditions reported in the literature in guinea pigs (Minarikova et al, 2015).
However, a challenging mismatch seems to exist between the conditions considered as common by the veterinary profession and those found in the literature (Robinson et al, 2017).
In part one of this article, gastrointestinal and urinary tract disorders will be taken into consideration. Refer to the literature provided for more details.
Anorexia
Anorexia is a common presenting complaint in pet guinea pigs. However, this is not a disease, but rather a non-specific symptom that may have several possible causes – from metabolic diseases to systemic infections.
Some specific conditions should be considered in the diagnostic work-up of the inappetent pig. Guinea pigs may be very sensitive to dietary changes, but they can also develop gastrointestinal tract stasis from food impactions and bezoars. A lack of vitamin C may also lead to painful joints and mastication.
Any sort of pain – from pododermatitis to dental disease and urolithiasis – may also induce inappetence.
Unfortunately, it is not always possible to detect antemortem the true cause of anorexia and subsequent weight loss in guinea pigs. However, all these cases should be fully investigated, and should have full body and skull radiographs, blood tests (haematobiochemistry) and urinalysis.
Treatment is initially supportive, and should include replacement fluids (maintenance rate: 80ml/kg/day to 100ml/kg/day), vitamin C supplementation (50mg/day to 100mg/day), syringe feeding (a variety of diets specifically formulated for recovering herbivores can be found commercially) and analgesia as needed. The primary cause should be identified and treated accordingly.
Gastrointestinal diseases
Dental disease
Dental disease is one of the most common reasons for presentation of guinea pigs to the veterinary practice.
Guinea pigs are very popular hystricomorph rodents and, as such, are characterised by a specific development of the masseter muscle (Cox et al, 2012), which results in an effective grinding action at the molars.
This is a very important consideration as the normal dental anatomy of rodents closely relates to their feeding habits (Yarto-Jaramillo, 2011).
Guinea pigs have long crowned (hypsodont), open-rooted (aradicular) incisors and cheek teeth, which continuously grow (elodont) several inches annually, leading to malocclusion if they are not worn down sufficiently (Legendre, 2002).
The primary cause of dental disease in guinea pigs is under debate (Lennox et al, 2020). Inappropriate diet, hypovitaminosis C, infections and trauma may all play a role in the development of dental disease in this species.
Secondary nutritional hyperparathyroidism with fibrous osteodystrophy has been reported in guinea pigs of the satin breed with dental problems (Jordan et al, 2009).
Primary incisor malocclusion is considered rare in guinea pigs.
Incisors’ abnormalities are more often the result of dental disease of the cheek teeth (Legendre, 2016). Metabolic bone disease has not yet been described as a cause for dental disease in this species.
Clinical signs are often subtle and non‑specific, and early identification of dental issues may be particularly difficult in this species compared to rabbits and other rodent species. Guinea pigs also appear to be less tolerant of dental changes than other species.
Oral-conscious examination is challenging and needs to be followed by adequate assessment under anaesthesia (Figure 1). Knowledge of the peculiar dental anatomy is essential to detect subtle changes and to understand the typical pattern of malocclusion in this species.
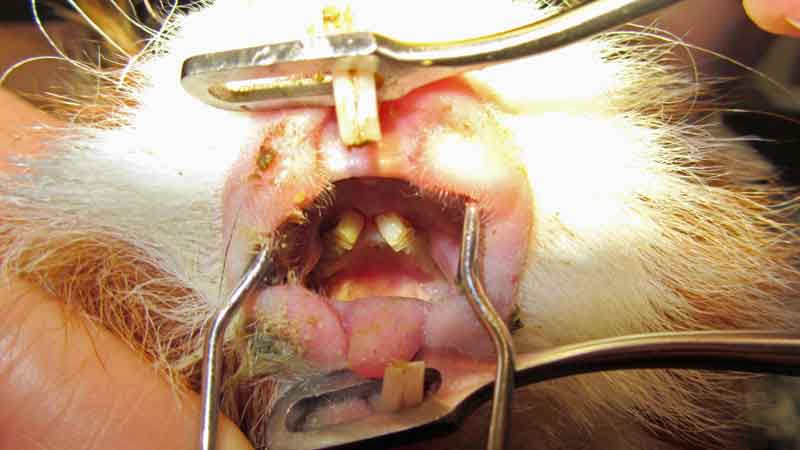
Because of the orientation of the cheek teeth, mandibular clinical crowns always elongate lingually, and maxillary clinical crowns elongate buccally. Crown overgrowth is not always as evident and spur formation is much less common than in rabbits (Lennox et al, 2020).
Definitive diagnosis can be achieved by a combination of imaging modalities, such as stomatoscopy, radiography and CT. Treatment follows directions already published for other species, such as rabbits, but is often complicated by the poor clinical conditions of the animal presented and the peculiar anatomy of this species.
Coronal reduction, teeth extractions and abscess surgery follow general principles, and have been reviewed elsewhere (Legendre, 2016; Lennox et al, 2020).
Gastric stasis, dilation and torsion
Gastrointestinal (GI) hypomotility or stasis is secondary to virtually any disease causing pain or anorexia in guinea pigs (Hawkins and Bishop, 2012). Inadequate dietary fibre content also represents a significant predisposing factor potentially leading to GI hypomotility, dehydration of GI content and stasis (Hawkins and Bishop, 2012).
Clinical signs may include decreased or absent faecal output, anorexia, abdominal pain, hunched posture, distended (with gas or fluid) stomach and/or bowel loops, and reduced gut sounds. The GI content may become dehydrated, exacerbating pain and anorexia, and potentially leading to partial or complete GI obstruction (Hawkins and Bishop, 2012). GI stasis can also result in accumulation of a large amount of gas within the stomach and intestine.
Because guinea pigs cannot vomit or eructate, gastric dilation and bloat can occur, which may become life-threatening. Gastric dilatation‑volvulus has also been reported in guinea pigs (De Voe, 2001; Mitchell et al, 2010; Dudley and Boivin, 2011).
Several potential predisposing factors for gastric dilatation-volvulus in guinea pigs have been identified. A report suggested that gastric volvulus may also develop secondary to conditions resulting in delayed gastric emptying, such as gastric stasis disorder (Mitchell et al, 2010).
Anatomic abnormalities are not known to be associated with the condition in guinea pigs. The rotation of the stomach on its mesenteric axis results in functional obstruction of the oesophageal and/or pyloric sphincters and is associated with pronounced gastric distention with gas. Symptoms – similar to those seen in gastric stasis cases – may include abdominal distention (Figure 2), respiratory distress, anorexia, lack of faecal output, lack of gut sounds and sudden death.

Previous reports of gastric volvulus in guinea pigs have indicated varying degrees of rotation to 360°, with one case of 540° rotation reported (Dudley and Boivin, 2011).
Complications can be similar to dogs due to venous obstruction and production of endotoxins when torsion of the stomach and the mesenteric root occur.
Diagnosis is based on a combination of history, clinical signs, abdominal palpation and radiographs. On radiographic images, in cases of gastric dilation, the stomach will appear full and overdistended, and will usually have a gas bubble evident.
When mesenteric root torsion/volvulus occurs, the stomach will be either on the right side of the body or caudal to small intestinal loops (Figure 3).
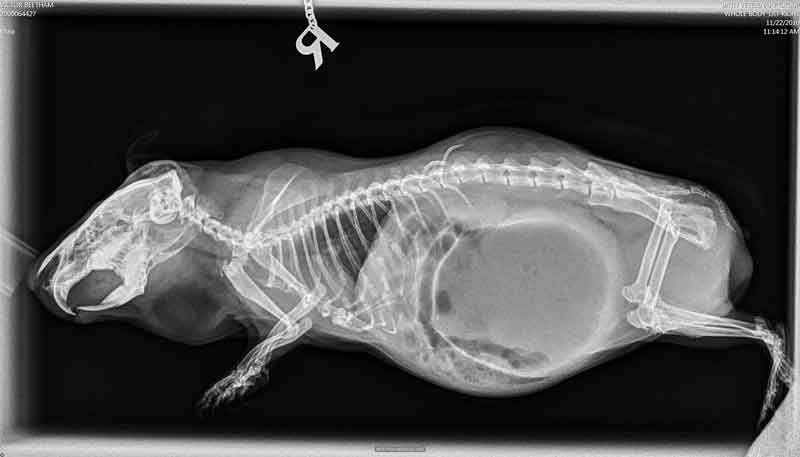
Gastric volvulus should be considered based on radiographic findings in a guinea pig exhibiting symptoms consistent with the condition. Confirmation usually occurs through exploratory surgery, or postmortem examination if death occurs.
This is an immediate surgical emergency with a very poor prognosis; however, reports exist of successful surgical correction of gastric dilation and volvulus with gastropexy (Kilgore, 2014).
Medical management includes aggressive supportive care with replacement IV fluid therapy, which is indicated in all cases of suspected gastric bloat. Gastroprotectants should always be considered. Avoid using prokinetics as partial or complete obstruction is generally present.
Gastric decompression is recommended on an emergency basis in severe cases of gastric tympany. A large bore red rubber tube with the end cut off is pre‑measured from the oral commissure to just beyond the final rib (Figure 4). Contents are usually a semi-solid mat of ingesta, so may be difficult to aspirate. The stomach should be gently palpated to determine when the pressure has been relieved.
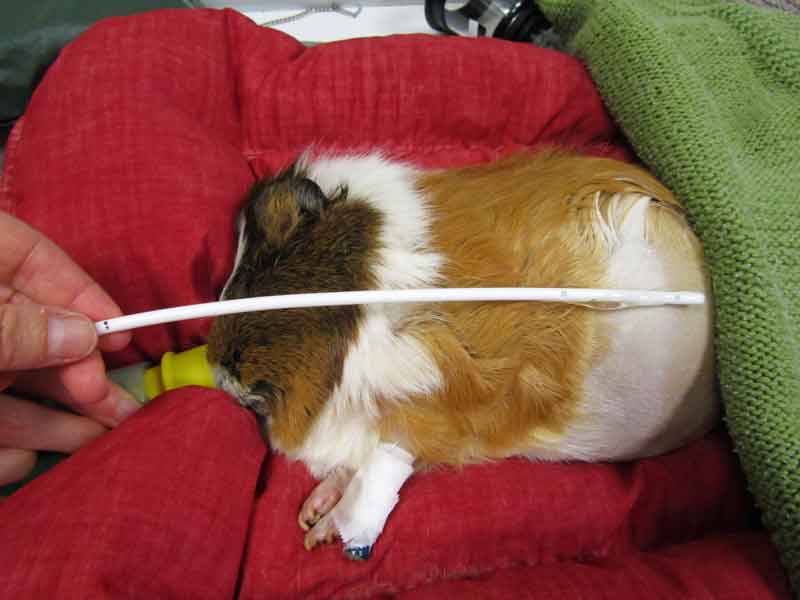
It is not necessary or recommended to completely empty the stomach. It is adequate to release the gas and relieve the distension.
Following this, fluid therapy and medical management can be used to further resolve the abnormality.
The patient should be further followed radiographically and kept hospitalised for 24 to 48 hours or until faeces are being produced and the patient is no longer distended (Figure 5).
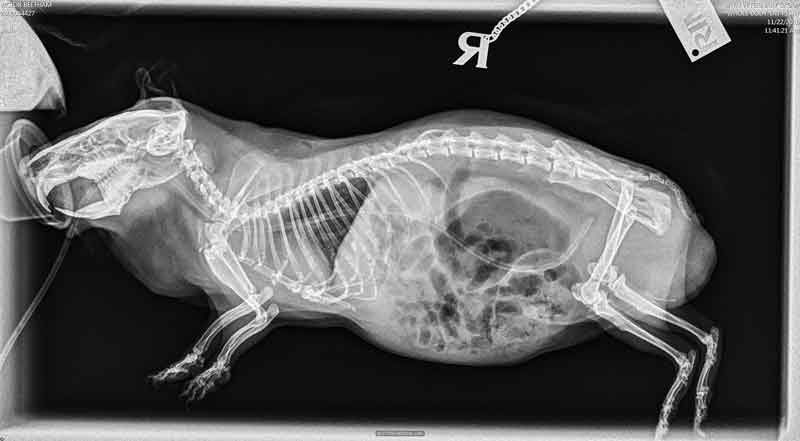
Guinea pigs with gastric displacement or mesenteric root volvulus are often euthanised as the prognosis is grave. Pain management is an essential component of the medical therapy.
Pain management is an essential component of the medical therapy (Pignon and Mayer, 2020).
Urinary tract disease
Urolithiasis
Urolithiasis most commonly affects middle‑aged to older guinea pigs (older than 2.5 years).
Stones may be found anywhere along the urinary tract – from the bladder, urethra, ureter, and kidneys (Figures 6 to 8) – and sometimes in the seminal vesicles of males (Hoefer and Latney, 2009; Hawkins and Bishop, 2012). A case of urethral diverticulum with an intraluminal urolith has also been reported (Parkinson et al, 2017).
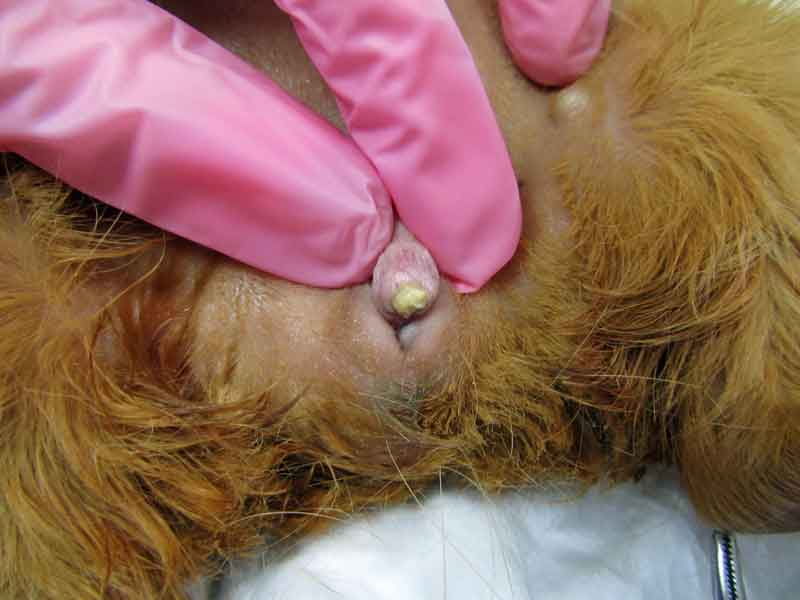
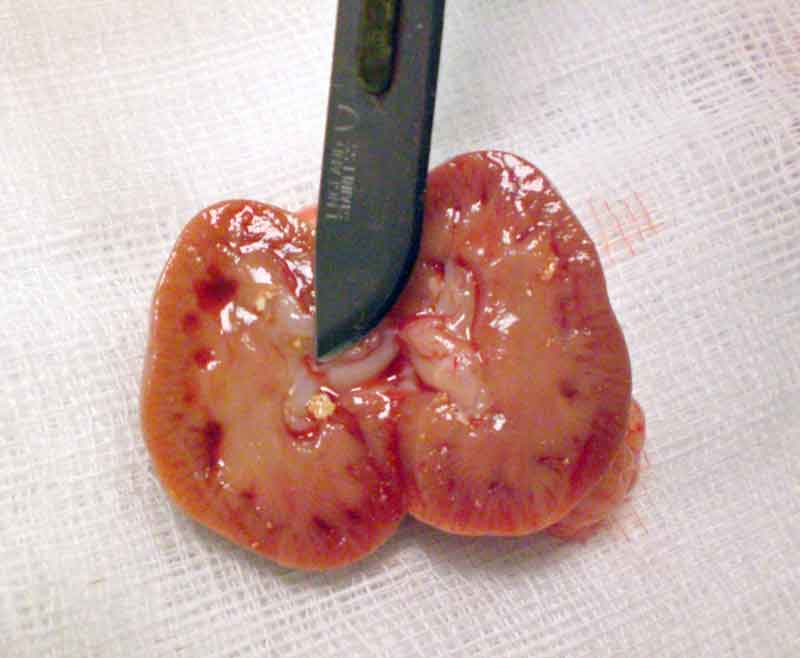
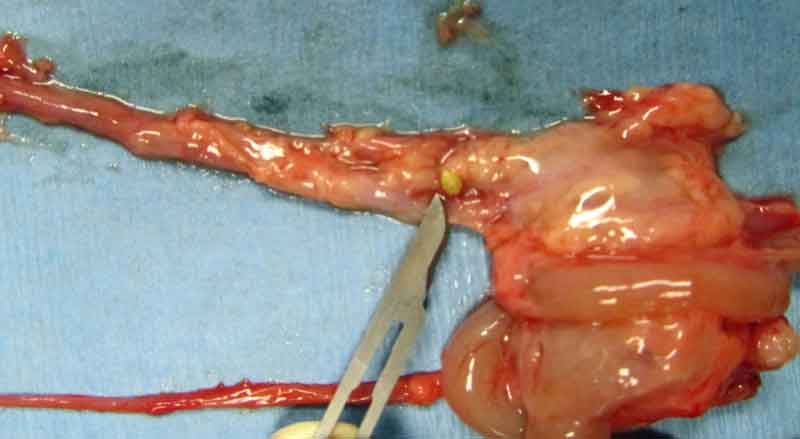
Many reports of this condition exist in the literature (Spink, 1978; Stuppy et al, 1979; Fehr and Rappold, 1997; Stieger et al, 2003; Eshar et al, 2013).
Its aetiopathogenesis remains unclear, with several risk factors identified (Hawkins, 2011; Hawkins et al, 2008). Many believe the alkaline pH and the high mineral content of normal guinea pig urine may favour crystal formation and precipitation (Hawkins and Bishop, 2012).
Risk factors contributing to urolithiasis may also include inadequate water intake, urine retention, inadequate cage hygiene, obesity, lack of exercise, food items with high mineral content (such as calcium and oxalate), renal disease and excessive vitamin or mineral supplementation (Hawkins, 2011). Diet also seems to play a major role, mainly with regards to calcium and oxalate levels.
In a study that looked into risk factors associated with development of urolithiasis, affected guinea pigs were more likely to be fed a diet high in overall percentage of pellets, low in percentage of hay, and with a lesser variety of vegetables and fruits (Hawkins et al, 2008). Calcium‑containing stones such as calcium oxalate (historically) and calcium carbonate (93%) are most commonly reported (Hawkins et al, 2008; 2009; Osborne et al, 2009). Infections and mechanical factors may also predispose to stone formation.
Symptoms generally depend on the size and location of the stone(s). Haematuria, stranguria and dysuria may be seen with cystic or urethral calculi. More commonly, clinical signs may reflect abdominal pain and may be seen more frequently when calculi are higher in the urinary tract.
Although presentation may be acute, it is common for signs to progress over days to weeks in affected rodents. A detailed history, including dietary history, is extremely important in association with a complete physical examination. A blood and urine sample should be collected for complete haematobiochemistry and urinalysis, to rule out kidney involvement and the presence of infection.
Healthy guinea pigs’ urine can undergo marked physiological visual changes in turbidity and colour within a period of 72 hours. This variability is reported as dependable on feeding, porphyria and physiological crystalluria (Rueloekke et al, 2016a). In a study, the median value for urinary protein to creatinine ratio (UPC) was found to be 1.60 (range 0.00 to 15.90). No sex‑related difference existed (p= 0.11), nor correlation between the age and UPC (r2=0.00 and p=0.76; Rueloekke et al, 2016b).
These findings indicated that either subclinical renal disease is a frequent condition in guinea pigs or physiological proteinuria of non-renal origin is a common finding in voided guinea pig urine. Therefore, UPC seems inapplicable as a diagnostic parameter for suspected renal disease in this species.
Culture of the urine collected in a sterile way (prior to antibiotic use), or of the bladder wall can direct the antibiotic choice when the sediment examination reveals the presence of bacteria, red and/or white blood cells.
Abdominal radiographs are also valuable as urinary calculi in guinea pigs are generally radiopaque. Abdominal ultrasound can help to confirm a presumptive diagnosis, or it may be necessary to localise stones in the urethra, ureter or kidneys.
Ultrasonography also allows evaluation of changes within kidneys, ureters or bladder (such as hydronephrosis, hydroureter or mucosal inflammation), as well as other abdominal (non‑urinary) pathologies. Contrast cystography and urethrography may provide more specific information regarding the bladder and urethra. An IV pyelogram or excretory urography may permit further functional evaluation of the urinary tract. More recently, ultrasound‑guided percutaneous antegrade pyelography has been described as a useful and easy-to-perform diagnostic technique in guinea pigs with hydronephrosis and hydroureter (d’Ovidio et al, 2020).
Alternatively, detailed images can be obtained with a CT scan and contrast agents can be used to elucidate functional abnormalities.
Cystoscopy may also be a useful diagnostic tool for direct visualisation of the mucosal surface of the urethra and urinary bladder, and allows obtaining biopsies of mucosal lesions (Wenger and Hatt, 2015).
Because the exact mechanism of stone formation is not known in guinea pigs, clear directions for prevention and dissolution of calculi cannot be given in this species, and surgical removal of the urinary calculi is considered the treatment of choice (Hawkins and Bishop, 2012).
Recurrence of the condition once the calculi have been surgically removed is high; owners should be advised and informed accordingly.
Dietary modification, vitamin C supplementation, supportive and symptomatic treatment (including appropriate hydration, adequate pain control, assisted feeding and antibacterial treatment, when indicated and following appropriate testing) are essential parts of the medical management plan in these cases.
A case series of 16 female guinea pigs undergoing urethrolith removal directly from the urinary orifice or urethra, with the aid of a lightweight self-retaining retractor ring with elastic stays, has been recently described (Lewis and Lennox, 2020).
Cystoscopic urolith removal may also be a more rapid and less invasive alternative to surgical removal (Wenger and Hatt, 2015; Figure 9).
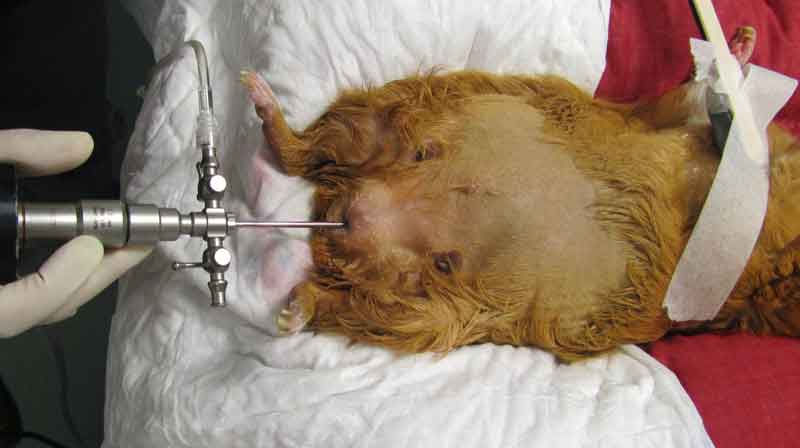
If this is not possible and the guinea pig is not obstructed, supportive care – including fluids and assisted feeding – should be provided, and the surgery for removal of the stone scheduled appropriately depending on the clinical conditions of the patient. Cystotomy is performed as routine (Figure 10).
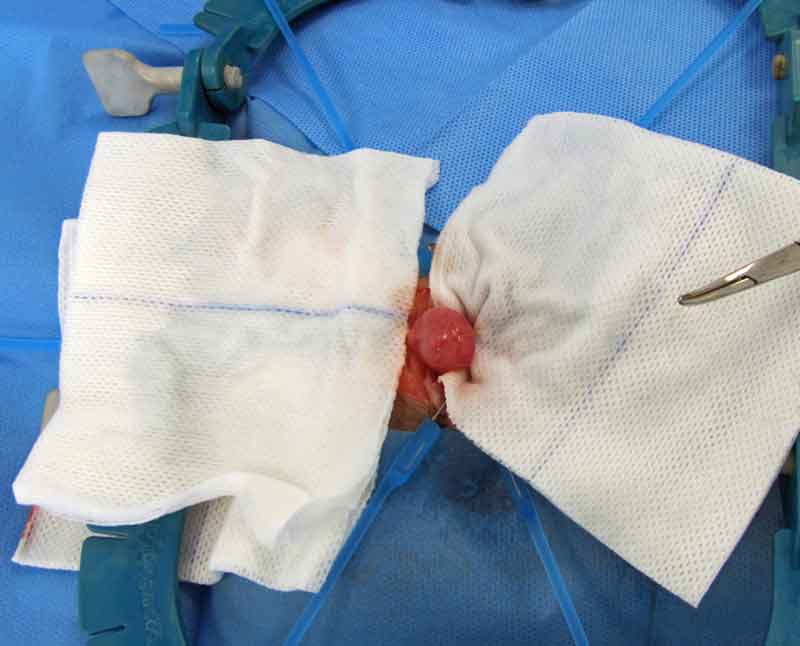
Surgery is also considered the preferred procedure for relief of ureteral obstructions.
Ureterotomy has been described in guinea pigs, but the significant complications following this procedure should be taken into consideration (Stieger et al, 2003; Bennett, 2012; Mancinelli, 2012; Figure 11).
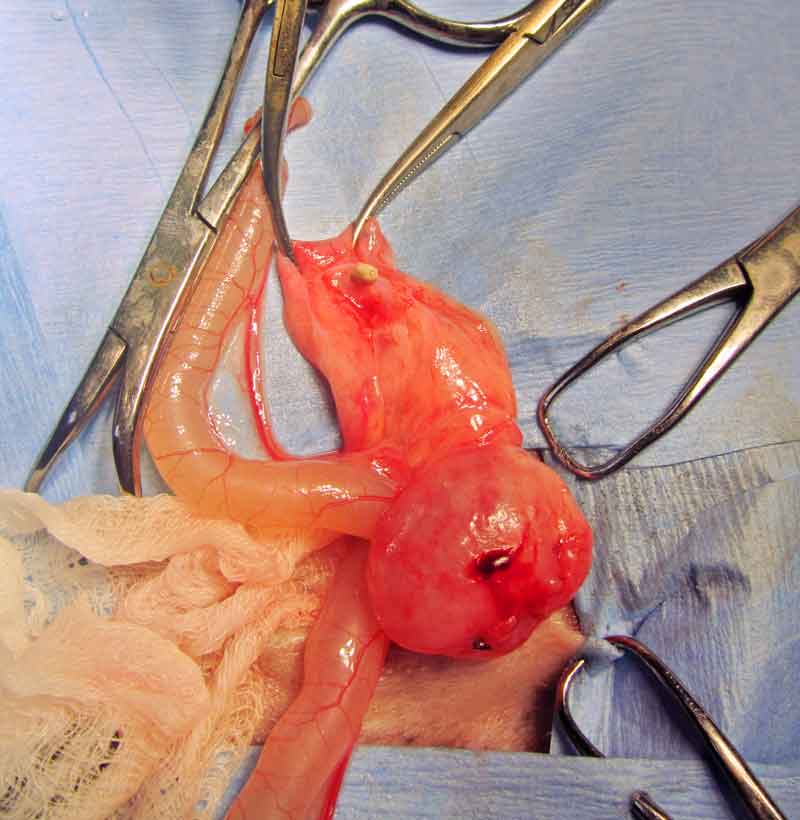
The palliative clinical management of a complicated ureteral obstruction in a guinea pig has been described using an ultrasound-guided percutaneous anterograde nephrocentesis and hydropropulsion (Eshar et al, 2013). Staged procedures may also be considered (Fawcett et al, 2016).
References
- Bennet RA (2012). Soft tissue surgery. In Quesenberry KE and Carpenter JW (eds), Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery (3rd edn), Elsevier, St Louis: 326-338.
- Cox PG et al (2012). Functional evolution of the feeding system in rodents, PLOS One 7(4): e36299.
- De Voe R (2001). Clinical snapshot: gastric bloat with possible volvulus in a guinea pig, Compend Contin Educ Pract Vet 23: 543-571.
- d’Ovidio D et al (2020). Ultrasound‑guided percutaneous antegrade pyelography for suspected ureteral obstruction in 6 pet guinea pigs (Cavia porcellus), Vet Q 40(1): 198-204.
- Dudley ES and Boivin GP (2011). Gastric volvulus in guinea pigs: comparison with other species, J Am Assoc Lab Anim Sci 50(4): 526-530.
- Eshar D et al (2013). Ultrasound‑guided percutaneous antegrade hydropropulsion to relieve ureteral obstruction in a pet guinea pig (Cavia porcellus), Can Vet J 54(12): 1,142-1,145.
- Fawcett A et al (2016). Surgical removal of uroliths from the urethra and urinary bladder of a geriatric female guinea pig, Aust Vet Pract 46(3): 88-91.
- Fehr M and Rappold S (1997). Urolithiasis in 20 guinea pigs (Cavia porcellus), Tierarztl Prax 25(5): 543-547.
- Hawkins MG (2011). Urinary tract obstruction. In Oglesbee BL (ed), Blackwell’s Five‑Minute Veterinary Consult: Small Mammals (2nd edn), Wiley Blackwell, West Sussex: 336-338.
- Hawkins MG and Bishop CR (2012). Disease problems of guinea pigs. In Quesenberry KE and Carpenter JW (eds), Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery (3rd edn), Elsevier, St Louis: 295-310.
- Hawkins MG et al (2008). Risk factors associated with the development of urolithiasis in pet guinea pigs (Cavia porcellus), Proc Annual Association of Avian Veterinarians Association of Exotic Mammal Veterinarians: 58.
- Hawkins MG et al (2009). Composition and characteristics of urinary calculi from guinea pigs, J Am Vet Med Assoc 234(2): 214-220.
- Hoefer H and Latney LT (2009). Rodents: urogenital and reproductive system disorders. In Keeble E and Meredith A (eds), BSAVA Manual of Rodents and Ferrets, BSAVA, Gloucester: 150-160.
- Jenkins JR (2010). Diseases of geriatric guinea pigs and chinchillas, Vet Clin North Am Exotic Anim Pract 13(1): 85-93
- Jordan J et al (2009). Clinical, radiological and laboratory investigation of osteodystrophia fibrosa in guinea pigs (Cavia porcellus) of the satin breed, Kleintierpaxis 54(1): 5-13.
- Kilgore A (2014). Gastropexy to manage recurrent gastric dilatation and volvulus in a guinea pig, Proc Association of Exotic Mammal Veterinarians, Orlando.
- Legendre LFJ (2002). Malocclusion in guinea pigs, chinchillas and rabbits, Can Vet J 43(5): 385-390.
- Legendre L (2016). Anatomy and disorders of the oral cavity of guinea pigs, Vet Clin North Am Exot Anim Pract 19(3): 825-842.
- Lennox AM et al (2020). Small mammal dentistry. In Quesenberry KE, Orcutt CJ, Mans C and Carpenter JW (eds), Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery (4th edn), Saunders, St Louis: 514-535.
- Lewis TT and Lennox AM (2020). Nonsurgical removal of urethral uroliths using a self-retaining retractor with elastic stays in female guinea pigs (Cavia porcellus): 16 cases (2006‑2019), J Exot Pet Med 36: 11-15.
- Mancinelli E (2012). Surgical treatment of urolithiasis in two guinea pigs, Proc BVZS Autumn Meeting, Edinburgh: 32.
- Minarikova A et al (2015). Diseases in pet guinea pigs: a retrospective study in 1,000 animals, Vet Rec 177(8): 200.
- Mitchell EB et al (2010). Gastric dilatation-volvolus in a guinea pig (Cavia porcellus), J Am Anim Hosp Assoc 46(3): 174-180.
- Osborne CA et al (2009). Quantitative analysis of 4,468 uroliths retrieved from farm animals, exotic species, and wildlife submitted to the Minnesota Urolith Centre: 1981 to 2007, Vet Clin North Am Small Anim Pract 39(1): 65-78.
- Parkinson LAB et al (2017). Urethral diverticulum and urolithiasis in a female guinea pig (Cavia porcellus), J Am Vet Med Assoc 251(11): 1,313-1,317.
- Pignon C and Mayer J (2020). Guinea Pigs. In Quesenberry KE, Orcutt CJ, Mans C and Carpenter JW (eds), Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery (4th edn), Saunders, St Louis: 270-297.
- Robinson NJ et al (2017). Veterinarian nominated common conditions of rabbits and guinea pigs compared with published literature, Vet Sci 4(4): 58.
- Rueloekke ML et al (2016a). Physiologic Serial Changes in the Visual Appearance of Urine Samples from Individual Pet Guinea Pigs (Cavia porcellus), Proc BSAVA Congress, Birmingham: 553-554.
- Rueloekke ML et al (2016b). Establishment of normal values of urine protein/creatinine ratio in pet guinea pigs (Cavia porcellus), Proc BSAVA Congress, Birmingham: 554.
- Spink RR (1978). Urolithiasis in a guinea pig (Cavia porcellus), Vet Med Small Anim Clin 73(4): 501-502.
- Stieger SM et al (2003). Ureterolithiasis and papilloma formation in the ureter of a guinea pig, Vet Radiol Ultrasound 44(3): 326-329.
- Stuppy DE et al (1979). Urolithiasis and cystotomy in a guinea pig (Cavia porcellus), Vet Med Small Anim Clin 74(4): 465-467.
- Wenger S and Hatt JM (2015). Transurethral cystoscopy and endoscopic urolith removal in female guinea pigs (Cavia porcellus), Vet Clin North Am Exot Anim Pract 18(3): 359-367.
- Yarto-Jaramillo E (2011). Respiratory system anatomy, physiology and disease: guinea pigs and chinchillas, Vet Clin North Am Exot Anim Pract 14(2): 339-355.
