23 Jun 2020
Critical care of tortoise found in pond
Violaine Colon details the case of a 60-year-old male that was unresponsive and apnoeic after being recovered from this location.

Image: © pedro / Adobe Stock
A 60-year-old Hermann’s tortoise was found at the bottom of a pond after being missing for two days. The reptile was unresponsive and apnoeic on arrival to the clinic. Critical care was investigated (intubation, assisted breathing and warming the patient to the preferred optimal temperature zone) and spontaneous breathing resumed. After a few days of hospitalisation, the tortoise made a full recovery.
This case report confirms that reptiles have the capacity of surviving a long period of apnoea.
Tortoises with free access outdoors are at risk of various accidental injuries.
This case illustrates an instance where a tortoise had been recovered from a pond. Treatment is discussed along with the challenge in this species of determining whether a tortoise is dead.
Signalment
A 60-year-old male Hermann’s tortoise (Testudo hermanni) weighing 1.1kg was presented.
History
The tortoise had been missing for two days. It was found at the bottom of a pond in the owner’s garden.
Husbandry
The tortoise was normally kept in the garden, with no access to a heat lamp or UV light. Hibernation was allowed every year. Diet consisted mainly of fresh leafy greens and the tortoise was allowed to graze in the garden.
Clinical examination
On admission, the tortoise was cold, unresponsive and not breathing. A heartbeat was present at 28 beat/min and slight reflexes (corneal and withdraw reflexes) were present.
Otherwise, the tortoise was in good body condition, and the rest of the clinical examination was unremarkable. See Panel 1 for how to know if a tortoise is dead.
Brain death can be challenging to identify in chelonians.
The criteria on which a diagnosis of brain death can be made includes “absolute unresponsiveness to external stimuli; cessation of movement and breathing, including no spontaneous breathing for three minutes after an artificial respirator has been turned off; and complete absence of cephalic reflexes. The pupils of the eyes must be dilated and unresponsive to direct light” (Studdert et al, 2006).
Practical methods:
- Warm the patient
- Assess reflexes: corneal reflex, withdraw reflexes, jaw tone, limb rigidity.
- Check heart with Doppler.
- A heart rate less than 15 beats per minute carries a poorer prognosis (Bonner, 2000).
- The heart can still be beating for a couple of hours after brain death.
- If the tortoise is dead, the heart rate will slowly decrease over two to three hours.
- If unsure, keep in a warm environment (about 25°C) for a couple of hours; if still unresponsive then death can be pronounced.
Treatment
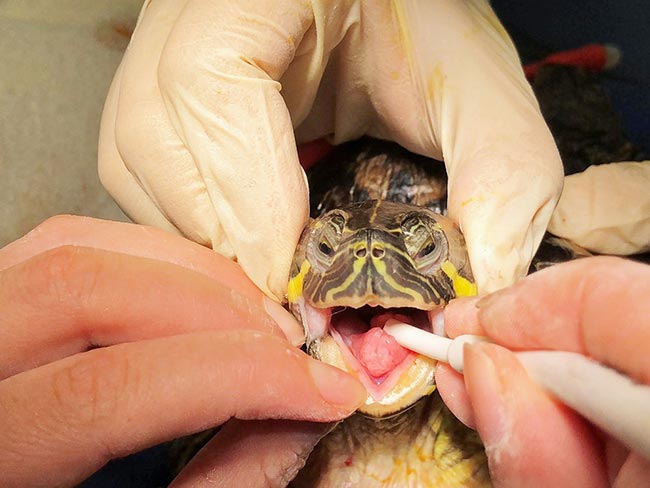
The tortoise was intubated (Panel 2) and connected to intermittent positive pressure ventilation to ventilate with oxygen at a breathing rate of five breaths per minute.
- The trachea bifurcates at the level of the thoracic inlet, so a short tube (1mm to 3mm) is needed.
- Complete cartilaginous tracheal rings, so use an uncuffed tube.
- The glottis is at the base of the tongue and will be closed while the tortoise is not breathing.
The tortoise was warmed by using an air blanket and warmed intracoelomic fluids (Hartmann‘s solution) were given to correct daily requirements (10ml/kg/day to 30ml/kg/day).
The tortoise was then monitored for the next 12 hours and kept hospitalised in a room with an ambient temperature of 24°C, and under a heat lamp at 30°C.
After a few hours on the ventilator, manual breathing was performed with room air using a bag valve mask.
The main stimulus to breathe in reptiles is low levels of oxygen in the blood (Longley, 2008). Overventilation can cause further respiratory depression by raising the partial pressure of oxygen (Long, 2016).
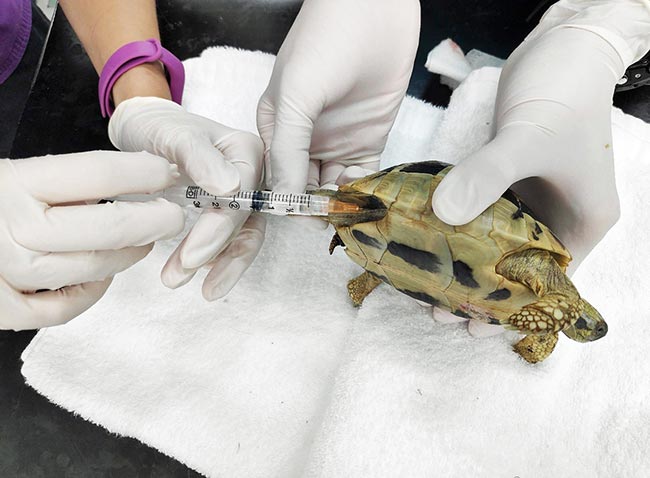
After five hours, the tortoise started breathing on its own and was extubated. The next morning, it was reactive, bright and alert. The tortoise was started on a course of prophylactic antibiotic (ceftazidime 22mg/kg every 72 hours) by IM injection to cover for possible aspiration pneumonia.
The tortoise was kept hospitalised for the next couple of days and continued to improve. Supportive care (fluids and assisted feeding with a critical care herbivore fine grind recovery food at a dose of 20ml/kg; Norton, 2005) was provided. It started eating dark green vegetables after 48 hours.
Blood test for haematology and biochemistry were performed to check for any underlying conditions, which did not reveal any abnormalities.
Radiology was not performed as, in acute cases, lung changes are unlikely to be visible (Chitty and Raftery, 2013). In more chronic cases, lung consolidation (caused by fibrosis, neoplasia, inflammation or infection) can be observed as an increase in lung opacity (Hernandez-Divers, 2006).
After five days of hospitalisation, the tortoise was discharged with ceftazidime injections to continue the antibiotic course.
Outcome
At a follow-up appointment, the tortoise was doing well.
Discussion
How can a tortoise survive for so long immersed in water?
Chelonians have the ability to lower their metabolism (Chitty and Raftery, 2013). They can also switch to dive reflex, anaerobic respiration and cardiac shunting, which allow them to tolerate hypoxia (Schumacher, 2003; O’Malley, 2005).
Long (2016) wrote: “Apnoea, bradycardia, diving or lung disease can increase pulmonary resistance, inducing the right‑to‑left cardiac shunting and directing blood flow from the right to left ventricles, bypassing the lungs. The physiologic advantage of shunting is that it allows the continual circulation and delivery of oxygen to the tissues during apnoea.”
This explains how tortoises have a high survival rate after falling into ponds, even after a few hours.
What do you do when you are presented with an unresponsive tortoise?
When presented with an unresponsive tortoise, apply the following steps.
Warm the tortoise
Warm the tortoise slowly to the preferred optimal temperature zone (POTZ) temperature (25°C to 30°C for most Mediterranean species).
Chelonians are ectothermic and depend on the environmental temperature to maintain their body temperature. POTZ is the temperature range where the tortoise can function optimally overall (Norton, 2005). Temperature will influence heart rate, respiratory rate and physiological functions. Heart rate will increase with temperature (Longley, 2008).
Warmth can be provided by different methods, such as:
- incubator
- brooder
- heat mat
- forced-air blanket
- heat bulb
- water bottle
Temperature should be measured – especially if the tortoise is not responding – and care should be taken not to overheat the patient.
Assess the patient
Principles of resuscitation are the same as other animals: airway, breathing and circulation.
- Breathing:
- Intubate (Panel 2) and manually ventilate or connect to mechanical ventilator if not breathing.
- If cannot intubate, move the front legs to breathe for the tortoise, as chelonians do not have a diaphragm, and use their strong trunk muscle to expand and contract their lungs (O’Malley, 2005).
- Administer doxapram at 4mg/kg to 12mg/kg IM, IV or by mouth once to stimulate breathing (Meredith, 2015).
- Monitor heart rate with a Doppler placed on the side of the neck:
- Normal heart rate is variable depending on the temperature of the tortoise and the size. For a 1kg Mediterranean tortoise kept at the ideal POTZ (20°C to 28°C), the heart rate is 40bpm to 60bpm (Redrobe, 2004).
- Assessing hydration status:
- Clinical signs of dehydration include “sunken eyes; changes in skin turgor; skin tenting; loss of skin suppleness; dry mouth with ropey, thick oral secretions; depression; a slow and difficult to find heart beat; and minimal to no urination” (Norton, 2005).
More details on how to treat critical tortoise can be found in the literature.
- Drugs mentioned in this article are used under the cascade.
