1 Nov 2016
Diagnosing endocrine disease in parrots
While disorders of parrots' endocrine systems are rare, Yvonne van Zeeland insists the principles of diagnosis and treatment in mammals are a good starting point in suspected cases.

Compared to mammals, birds, including parrots, seem to seldom suffer from endocrine disorders.
Hypoadrenocorticism and hyperadrenocorticism, as well as hyperthyroidism and thyroid neoplasia, have rarely been recognised in live birds. Hypothyroidism is suggested to occur more often, but, in many cases, lack of appropriate testing prevents a definite diagnosis from being made, thereby keeping the number of confirmed cases low.
Secondary nutritional hyperparathyroidism, on the other hand, is among the most common endocrine diseases seen in parrots, particularly in those fed diets low in calcium and/or vitamin D. Similarly, a poor calcium:phosphorus ratio may result in osteodystrophy or hypocalcaemia. Imbalanced diets may, furthermore, result in iodine deficiency and goitre, most commonly seen in budgerigars. Pituitary neoplasia and diabetes mellitus are also seen in this species, but may occur in other species.
In many cases, antemortem diagnosis and treatment of endocrine disease remains challenging because of the limited pathophysiological knowledge, unavailability of validated tests and lack of information on appropriate dosing regimens in birds. However, by remaining alert to the possibility of a potential underlying endocrine disorder, and using the knowledge obtained from other companion animals, endocrine disease in parrots may be more readily recognised, diagnosed and treated.
In birds, the endocrine system consists of the hypothalamic-pituitary complex and pineal gland, carotid bodies, the thyroid, parathyroid, ultimobranchial and adrenal glands, the pancreas and endocrine cells of the gastrointestinal tract, and the gonads (Ritchie and Pilny, 2008; Orosz, 2016).
All of these organs secrete hormones into the bloodstream that help to maintain homeostasis and regulate internal processes, such as growth and development, metabolism, and reproductive activity.
Disease usually occurs as a result of a hypofunction or hyperfunction of the gland.
Endocrine diseases do occur in psittacine birds, but their incidence appears to be relatively low – most of the available information is derived from single case reports or small case series.
However, the low incidence may, in part, be explained by the lack of knowledge and appropriate tests to diagnose endocrine abnormalities.
As a result, endocrine disease might occur more often and should not be ruled out when compiling a differential diagnosis.
Of the various endocrine diseases that have been reported in parrots, secondary nutritional hyperparathyroidism, resulting in hypocalcaemia and/or metabolic bone disease, appears most common, especially in birds fed an all-seed diet (Stanford et al, 2013).
Similarly, goitre may be seen, most frequently in budgerigars, as a result of iodine deficiency (Schmidt, 2002). Other endocrine diseases appear less common in parrots, with reports of their incidence being limited to a single or small number of birds.
Pituitary gland diseases
The pituitary gland, also referred to as the hypophysis, is at the base of the brain, just below the hypothalamus.
It consists of an anterior part (adenohypophysis) and posterior part (neurohypophysis) that both produce and secrete hormones, such as (Ritchie and Pilny, 2008; Orosz, 2016):
- luteinising hormone [LH]
- follicle stimulating hormone [FSH]
- thyroid stimulating hormone [TSH]
- adrenocorticotropic hormone [ACTH]
- growth hormone [GH]
- prolactin by the adenohypophysis arginine vasotocin [AVT]
- mesotocin by the neurohypophysi
Disease may occur as a result of various processes, most commonly as a result of a (benign) neoplastic growth.
Such pituitary tumours have most commonly been described in budgerigars (Schlumberger, 1954; Bauck, 1988; Langohr et al, 2012), but may also occur in other species (Curtis-Velasco, 1992; Latimer, 1994; Lumeij, 1994; Romagnano et al, 1995; Starkey et al, 2008).
Generally, clinical signs will develop as a result of the mass pressing against the surrounding tissues, thereby causing neurological signs, exophthalmos and/or blindness (De Matos and Monks, 2016).
Similarly, pressure against the normal pituitary cells may result in hyposecretion of one or more of the pituitary hormones. Occasionally, hypersecretion of a specific hormone may be noted, leading to clinical signs of, for example, Cushing’s disease (as a result of overproduction of ACTH; Starkey et al, 2008) or diabetes insipidus (as a result of overproduction of AVT; Starkey et al, 2010).
A presumptive diagnosis can be made based on the clinical signs and ruling out of other diseases, but definite diagnosis requires visualisation of the pituitary mass using advanced imaging techniques, such as CT or MRI.
In addition, hormonal hypersecretion may be detected using hormone-specific assays or function tests (for example, water deprivation test; Alberts et al, 1988; Lumeij and Westerhof, 1988; Starkey et al, 2010).
However, size of the patient and tumour may prevent antemortem diagnosis of the tumour, thereby rendering postmortem examination as the only option to obtain a final diagnosis.
Thus far, no treatment has been reported for these types of tumours, although one report has demonstrated long-term management of central diabetes insipidus in a parrot using desmopressin (Starkey et al, 2010).
Thyroid gland diseases
Disease of the thyroid gland may result from hyperplasia, neoplasia, infection, inflammation or atrophy, with clinical signs occurring as a result of enlargement, hypofunction or hyperfunction of the gland (De Matos and Monks, 2016).
The number of documented cases of thyroid tumours and hyperthyroidism in psittacine birds is scarce, with most cases involving non-functional thyroid adenomas or adenocarcinomas (Lumeij, 1994; Rae, 1995; Doukaki et al, 2014).
Clinical signs will usually result by the tumour compressing the surrounding tissue and may include (Lumeij, 1994; Rae, 1995; Doukaki et al, 2014):
- dyspnoea
- regurgitation
- dysphagia
These clinical signs may more commonly be observed, especially in budgerigars (Schmidt, 2002) in cases of thyroid hyperplasia (goitre). This disease, which is most often caused by an iodine deficiency, is usually diagnosed based on the history, clinical signs and response to dietary iodine supplementation (Lumeij, 1994).
Hypothyroidism has often been suspected as the cause of feather loss, due to a lack of feather growth, and obesity in parrots (Oglesbee, 1992; Kritchevsky, 2013; Figure 1). In many cases, diagnosis was based on the finding of low plasma T4 and/or a positive response to thyroxine supplementation (Lumeij, 1994; Oglesbee, 1992; De Matos and Monks, 2016).

However, definite diagnosis requires the use of a TSH stimulation test, with hypothyroid birds showing less than two-fold increases in plasma T4 levels compared to baseline (Lothrop et al, 1985a; Zenoble et al, 1985; Lumeij, 1994; Greenacre, 2011; De Matos and Monks, 2016).
In addition, scintigraphy may be helpful to diagnose hypothyroidism, but is not widely available (Harms et al, 1994).
Following confirmation of the diagnosis, treatment may be initiated with oral thyroxine supplementation (Oglesbee, 1992; De Matos and Monks, 2016).
Parathyroid gland diseases
Thus far, disease of the parathyroid glands in birds has primarily been linked to hyperplasia resulting from dietary calcium, phosphorus and/or vitamin D deficiencies or imbalances (Stanford et al, 2013).
These dietary imbalances will, subsequently, result in a disturbance of the mineral homeostasis, which stimulates the parathyroid glands to produce more PTH to correct these imbalances (Stanford et al, 2013).
Grey parrots appear particularly prone to developing disease, potentially resulting from an increased dependency on UV light for their calcium metabolism (Stanford, 2006; Stanford et al, 2013). Clinical signs often include:
- weakness
- ataxia
- tetany
- seizures
In young, growing birds, bone deformities and pathological fractures of the long bones and spinal column are frequently seen (Figures 2a and 2b), whereas female birds may present with egg binding, or production of poorly calcified or deformed eggs.

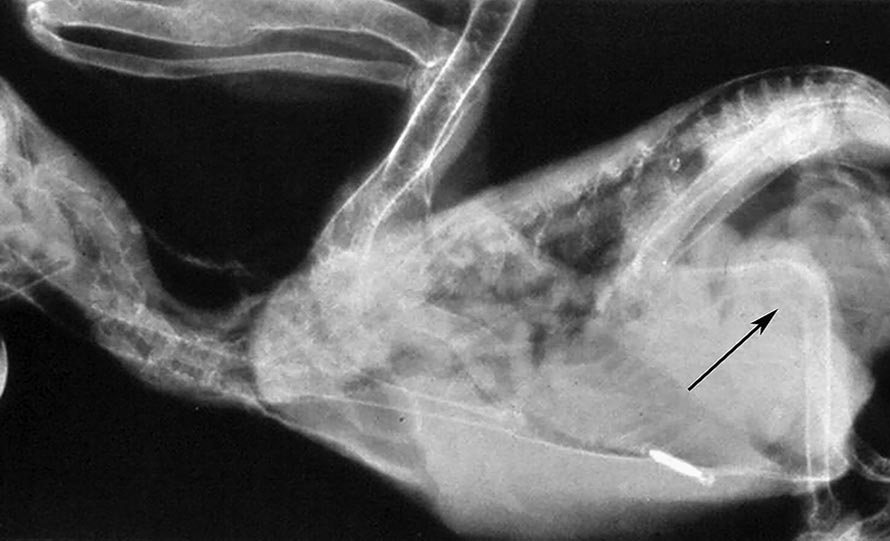
Diagnosis is usually based on low (ionised) calcium levels in the blood (Stanford, 2007), whereas radiographs may help diagnose bone abnormalities (Stanford et al, 2013; De Matos and Monks, 2016).
Treatment consists of oral and parenteral calcium and/or vitamin D3 supplementation, dietary corrections and exposure to UVB radiation through natural sunlight or artificial lighting (Stanford, 2004; Stanford et al, 2013).
Aside from hypocalcaemia, hypercalcaemia has been reported to occur in parrots. Most often, this is part of a physiological phenomenon in egg-laying females, with increased levels of oestrogen resulting in (Campbell-Ward, 2013):
- increased calcium absorption in the gastrointestinal tract
- increased production of calcium-binding proteins (such as albumin)
- mobilisation of calcium from the medullary bone
Pathological hypercalcaemia also occurs, occasionally as a result of paraneoplastic syndromes in case of lymphoma (de Wit et al, 2003), but most commonly in response to long-term provision of a diet containing excessive amounts of calcium and/or vitamin D3 (Schoemaker et al, 1997; Campbell-Ward, 2013).
The disease is commonly seen in young, growing birds, particularly macaws fed excessive amounts of vitamin and mineral supplements (Schoemaker et al, 1997; Campbell-Ward, 2013).
Clinical signs include (Schoemaker et al, 1997; Campbell-Ward, 2013):
- poor weight gain in growing animals
- depression
- anorexia
- nausea
- polyuria/polydipsia
- ataxia
- muscle weakness
- joint pain
Diagnosis is usually based on the clinical signs combined with high (ionised) blood calcium levels.
In addition, uric acid and/or vitamin D3 metabolites may be measured (Schoemaker et al, 1997; Campbell-Ward, 2013). Radiography, meanwhile, is often useful to assess bone density, renal size and detect tissue mineralisation (Schoemaker et al, 1997; Campbell-Ward, 2013).
In advanced cases, such as presence of tissue calcifications and visceral or articular gout, prognosis is generally poor.
In mild cases, prognosis may be more favourable following timely treatment with forced diuresis and dietary corrections (Schoemaker et al, 1997; Campbell-Ward, 2013).
Adrenal gland diseases
The avian adrenal glands differ from those in mammals with regard to the lack of a distinct cortex and medulla, and the production of corticosterone rather than cortisol as the predominant glucocorticoid hormone (Oglesbee, 1997; De Matos, 2008).
Similar to other animals, disease may result from hyperfunction or hypofunction of the adrenal glands, resulting in excess, or lack of, one of the adrenal hormones (glucocorticoids, mineralocorticoids, epinephrine, norepinephrine).
Thus far, the number of birds diagnosed with spontaneous hyperadrenocorticism has been scarce (Cornelissen and Verhofstad, 1999; De Matos, 2008; Starkey et al, 2008), with only one case with an antemortem diagnosis of Cushing’s syndrome as a result of a primary adrenal gland tumour reported in a psittacine bird (Van Zeeland et al, 2015).
In this Senegal parrot, clinical signs included:
- polyphagia
- polydipsia
- mild alopecia
- obesity
- hepatic lipidosis
Following diagnosis, which was achieved using a modified dexamethasone suppression test, the bird was successfully treated with trilostane for more than one year (Van Zeeland et al, 2015).
Aside from primary adrenal hyperfunction, hyperadrenocorticism may also result from overstimulation by a pituitary tumour or exogenous corticosteroid administration, resulting in similar signs as those described (Rae, 1995; Starkey et al, 2008).
In addition, exogenous corticosteroids exert profound effects on the immune system and may suppress both humoral and cell-mediated immunity, resulting in increased susceptibility to infections, in particular aspergillosis (Westerhof, 1997; Verstappen and Dorrestein, 2005; De Matos, 2008).
Thus far, there have been no reports documenting the presence of spontaneous hypoadrenocorticism in pet birds. It is, however, likely adrenal hypofunction occurs in these species.
Similar to other animals, adrenal cell atrophy or necrosis may be induced by neoplasia, bacterial or viral infections, immune-mediated disease, amyloidosis, trauma or drugs (for example, mitotane; Rae, 1995). Diagnosis may be achieved using an ACTH stimulation test (Lothrop et al, 1985b; Walsh et al, 1985; De Matos, 2008).
However, since the condition of animals with hypoadrenocorticism may quickly deteriorate as a result of hyponatraemia-associated dehydration and shock, and hyperkalaemia-associated cardiac disturbances, it is highly likely fatal complications develop too rapidly in birds without the possibility of obtaining an antemortem diagnosis or initiating appropriate treatment.
Diseases of the pancreas
In birds, glucagon, secreted by the alpha cells of the pancreatic islets, is considered the major glucose-regulating hormone, with insulin playing a less well-defined role in the glucose metabolism.
As such, glucagon excess, or inadequate insulin:glucagon ratios, rather than hypoinsulinaemia, has been proposed as the cause of avian diabetes (Sitbon et al, 1980; Hazelwood, 1984).
Histologically, destruction of beta cells (consistent with type-one diabetes in humans) has been documented in birds, with underlying causes varying from paramyxovirus or herpesvirus infections to hemochromatosis (iron storage disease; Gancz et al, 2007; Pilny, 2008).
Similarly, causes for peripheral insulin resistance (consistent with type-two diabetes), such as obesity, high levels of endogenous or exogenous corticosteroids or progestogens, and increased levels of other hormones (such as glucagon, growth hormone and epinephrine) have also been identified (Candeletta et al, 1993; Gancz et al, 2007; Pilny, 2008). Neoplasia associated with diabetes mellitus has been diagnosed in one bird (Ryan et al, 1982).
In parrots, diabetes mellitus is most commonly reported in budgerigars and cockatiels, although it may occur in larger species as well (Candeletta et al, 1993; Rae, 1995; Desmarchelier and Langlois, 2008; Gancz et al, 2007; Pilny, 2008; Campbell-Ward and Rand, 2013).
A presumptive diagnosis can usually be made based on the clinical signs (polyuria/polydipsia, weight loss despite good appetite; Figure 3) in combination with the finding of a persistent hyperglycaemia and glycosuria (Pilny, 2008; Campbell-Ward and Rand, 2013; De Matos and Monks, 2016).

The normal range for plasma glucose is significantly higher than in mammals and may range from 10mmol/L to 27.9mmol/L, dependent on the species, with values greater than 44.4mmol/L considered diagnostic for diabetes mellitus (Campbell-Ward and Rand, 2013).
Elevated fructosamine levels and/or ketonuria may also be found, supporting the diagnosis (De Matos and Monks, 2016). Furthermore, determination of plasma glucagon and insulin has been recommended, but remains challenging due to the lack of appropriately validated tests (Bonda, 1996; De Matos and Monks, 2016).
Treatment of birds with diabetes mellitus can be challenging, especially given the small size of many patients and the lack of clear-cut therapeutic protocols (De Matos and Monks, 2016).
Prior to initiating treatment with glucose-regulating drugs, attempts should be made to rule out and treat any underlying or concurrent conditions. Similarly, birds may require supportive care in the form of fluids or force feeding.
In birds with persistent hyperglycaemia, treatment may be attempted with insulin and/or oral hypoglycaemic agents (such as glipizide), although the success of this treatment may vary from case to case (Bonda, 1996; Gancz et al, 2007; Pilny and Luong, 2005; Campbell-Ward and Rand, 2013; De Matos and Monks, 2016).
Hospitalisation of the bird is often recommended to enable gradual titration and regular monitoring of the patient, including serial blood glucose monitoring and urinalysis to check for glycosuria, until the bird is stable, following which treatment can continue at home (Campbell and Rand, 2013; De Matos and Monks, 2016).
Conclusion
Disorders of the endocrine system appear to be relatively rare in parrots, with the exception of goitre and secondary nutritional hyperparathyroidism, which are both linked to malnutrition.
The incidence of these diseases is likely to decrease in the future due to the increased knowledge on dietary requirements and the higher percentage of people feeding their parrots a well-balanced diet.
In contrast, other, less recognised endocrine diseases may be diagnosed more frequently as a result of expansion of knowledge on avian medicine and availability of diagnostic tests in birds. At the same time, more experience can be gained with proper treatment regimens for endocrine disease in birds.
Until then, knowledge on diagnosis and treatment of endocrine diseases can be extrapolated from human literature and/or companion animal medicine and applied to birds.
However, it is important to realise not all the information obtained from mammalian species will be applicable to birds due to the presence of anatomical and physiological differences between the two groups.
Nevertheless, the basic principles of the diagnostic work-up and therapeutic intervention for endocrine diseases in mammals offer a good starting point to clinicians confronted with a parrot suspected of an endocrine disorder.
- Some drugs mentioned in this article are used under the cascade.
