1 Apr 2013
Diagnosis of otitis externa, media and interna in rabbits
Kevin Eatwell, in the first of a two-part article, looks at approaches to the clinical history and diagnosis of all forms of otitis presented in rabbits.
Aural disease in rabbits is becoming increasingly diagnosed in clinical veterinary practice and is often associated with chronic upper respiratory disease.

One of the common signs of otitis media and interna are vestibular disease and this is often erroneously considered to be due to Encephalitozoon cuniculi. Head tilt, nystagmus and torticollis are common presenting clinical signs in rabbits (Figure 1).
The most important differentials for this condition are E cuniculi causing central vestibular disease or Pasteurella multocida causing otitis media, which leads to otitis interna, and the clinical signs become evident due to pressure and disruption of the vestibular apparatus.
Presumptive therapy usually centres on treating these two different conditions. Many cases are treated with oral fenbendazole for the E cuniculi and the patient may be given antibiotics such as enrofloxacin. Nonsteroidals or, in some cases, steroids may be given. Supportive care measures are vital as severe cases are unable to feed and gastrointestinal stasis is inevitable. Euthanasia should be considered. Severe cases may require sedation to reduce the severity of any spiralling.
Vestibular disease is reported as a frequent manifestation of E cuniculi infection. However, to lead to such specific signs the inflammation created by the parasite must be in a single focus. This is unlikely and greater effort should be made to confirm that E cuniculi is, in fact, causing the clinical signs, primarily by ruling out the other most important cause – otitis media and interna. If both are negative, more unusual diagnoses may be relevant.

In peripheral vestibular disease, the vestibular nerve (cranial nerve VIII) and the labyrinth of the inner ear are affected, usually as an extension of otitis media. This could be due to simple pressure on the oval and round windows, inflammation or infection. These cases have a horizontal nystagmus, which can be positional or evoked.
Central vestibular disease is when the lesions are in the brainstem. These present with vertical nystagmus. This is more likely with encephalitis. Other nervous defects, such as paresis or reduced mentation, may be present if the disease is extensive or multifocal, which, of course, does not occur with otitis media and interna.
In a UK study, 52 per cent of rabbits had IgG antibodies to E cuniculi, indicating these rabbits had been exposed to this agent (Keeble, 2006). This result often leads to treatment of the rabbit with fenbendazole with no further diagnostics being performed to eliminate other causes. IgG antibodies elevate four weeks after infection, peak at nine weeks and then slowly decline. Paired titres may actually yield more useful information and are generally taken a month apart. An increased second titre suggests the infection is active. A negative IgG antibody does not rule out E cuniculi, as the infection may be early or latent.
IgM titres have become available and elevate quickly after infection and decrease to a low level by a month after infection. A high titre indicates recent active infection or a recent reactivation of a latent infection. This enables the clinician to differentiate between exposure and active (new or reactivated) infections.

Generally, IgG and IgM titres are run concurrently and compared. Approximately a third (32.8 per cent) of rabbits have IgM antibodies and this increases (to 54.4 per cent) if the rabbits have neurological disease (Jecklova, 2010). Given this, a large number of neurological rabbits may not have active E cuniculi infection, but it takes 70 to 100 days after infection for lesions to be identified in the CNS. High IgM titres would, therefore, not be expected in neurological cases unless a latent infection had been reactivated at the same time.
Antibody titres alone do not confirm the clinical diagnosis. One paper demonstrated that a cerebrospinal fluid (CSF) tap proved useful as there were elevated white cell counts (lymphomonocytic pleocytosis) if encephalitis was present (Jass, 2008). This could be due to any CNS inflammation and ruling out other CNS diseases such as toxoplasmosis is important. Laparoscopic biopsies may aid diagnosis, yielding signs of lymphocytic inflammation. Although urine PCR has become available, shedding only occurs for five weeks post-infection. Other less common differentials for nervous diseases include toxoplasmosis, herpes simplex, listeriosis or other degenerative CNS lesions (Keeble, 2011).
Aural anatomy in rabbits
Knowledge and experience of treating otitis in dogs and cats is an important prerequisite for treating otitis in rabbits. However, rabbit aural anatomy, disease pathogenesis and treatment are different.

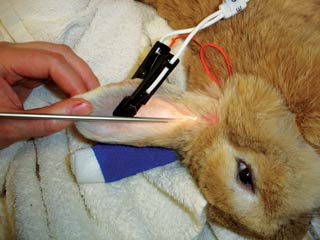
The external ear is made of a pinna and a vertical canal. The pinna has a central artery with many veins laterally, any of which can be catheterised if large enough, but most usually the caudal vein is utilised. The central ear artery is usually avoided for catheterisation, but can easily be used for blood gas analysis (Figure 2). Neovascularisation (to bypass previous sites of catheterisation) is very common. Pinna size is variable. Many breeds have an upright pinna, but lop breeds have a pinna that is folded downwards. Rabbits do not have separate vertical and horizontal canals, but have multiple cartilaginous plates making up the single “vertical” ear canal that extends dorsally from the bony acoustic meatus. This leads to the tympanic membrane at the ventral aspect of the meatus at the point at the entry to the bulla. The bulla consists of very thick bone laterally and thinner bone ventrally and projects ventral and lateral to the base of the acoustic duct (Figure 3). The bulla is 5mm deep, 7.5mm high and 11mm long (Chow, 2011; Mayer, 2011; Popesko et al, 1992).
Lop breeds are predisposed to otitis due to their altered anatomy (Chow, 2011; Capello, 2004). In these breeds they have a fold in the vertical canal between these plates and the canal is stenotic.
Rabbits have a much wider mandible than dogs and cats at its caudal extent. This extends ventrally in a semicircular fashion. This reduces the ability to palpate the bulla in a conscious rabbit. The angular process of the mandible protrudes caudally just below the entrance point to the bulla and provides a useful landmark (Figure 4).
Clinical signs associated with ear disease in rabbits
Otitis externa
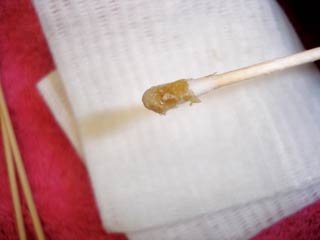
Clinical signs of otitis externa include scratching at the base of the ear, head shaking, pain, lethargy or anorexia. Primary bacterial infection or dermatopathies leading to otitis externa are rare. Rabbit wax is thick and appears similar to pus. However, wax is typically yellow or beige in colour (Figure 5). Pus is typically a white or creamy colour. Cytology can be used to confirm if there are large numbers of white cells or bacteria if otitis externa is present. Psoroptes cuniculi is a common parasitic problem and the diagnosis is essentially clinical (Figure 6). However, skin scrapes can be taken to confirm infection.
Otitis media
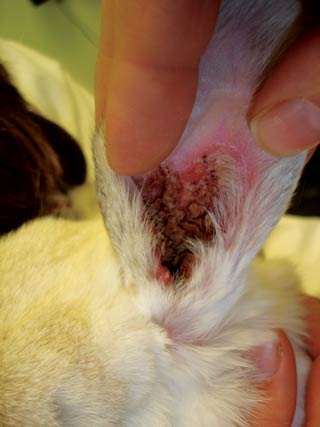
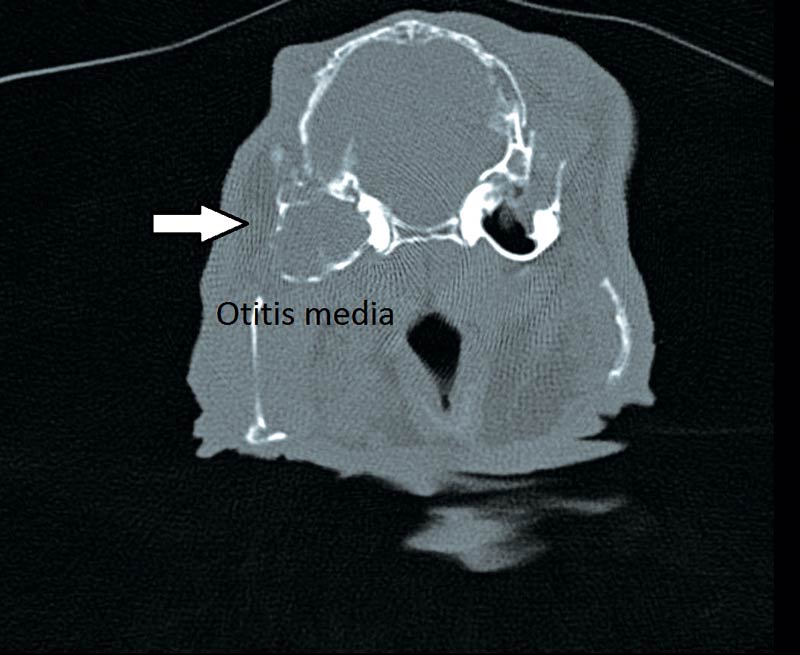
Otitis media cases are often clinically silent, but may be identified on imaging for another reason. These cases often present with lethargy, inappetence, pain, pruritus associated with the base of the ear, a head wobble, or a painful swelling at the base of the ear (Figure 7). Owners may report hearing deficits, but this is unusual.
Facial paralysis or spasticity may occur on the side of the lesion (Figure 8) due to extension of the pathology to involve the nerve around the lateral side of the bulla and ventral to the ear canal. Infection can extend to the external ear canal if the tympanic membrane has been ruptured. Alternatively, infection can progress from otitis external to otitis media. Unilateral and bilateral disease is seen. Otitis media may extend to otitis interna.
Otitis interna

Otitis interna is usually present with otitis externa. It can occur due to pressure, inflammation or infection. Head tilt, nystagmus, ataxia and circling are common signs. Facial nerve spasticity, paresis or deficits, may be present as the facial nerve exits ventral to the vestibulocochlear nerve in the internal acoustic meatus. Drooping of the upper lip, eyelid and ear (Horner’s syndrome) may be present and mimic facial paralysis.
Pathogenesis of otitis externa
Ceruminous gland hyperplasia does not occur, even with severe otitis externa. In cats and dogs this hyperplasia leads to narrowing of the ear canal. These changes are primarily in the vertical canal and so a lateral wall resection may be indicated in these species.
Cases of otitis externa in rabbits can be an extension of otitis media via the ruptured tympanic membrane, resulting in infection at the base of the vertical canal.
In severe cases, the local tissues can become involved and degeneration of the ear canal leads to a soft tissue swelling at the base of the ear canal. The infection rarely advances up the canal and it does not lead to marked histological changes of the dorsal section of the vertical canal.

Mild cases have minimal histopathological changes limited to subtle and nonspecific changes, such as hyperkeratosis or mild sebaceous ductal ectasia in the vertical canal (Figure 9). It is difficult to distinguish between these two disease processes clinically, but otitis media extending to otitis externa is by far the most frequent presentation.
Pathogenesis of otitis media or interna
Those cases with clinical signs of upper respiratory tract disease or those that are positive for Pasteurella multocida are highly likely to suffer from this condition due to infection ascending via the Eustachian tubes.
In one rabbit study, P multocida was isolated in most clinical and subclinical cases of otitis media and interna. However, isolation of P multocida does not equate to disease and up to 95 per cent of rabbits may be positive (Deeb et al, 1990; Snyder et al, 1973; Smith and Webster, 1925).
In dogs, culture results yielded from tympanic bullae pre and post-flushing at surgery demonstrated a 33 per cent reduction in isolates, but 70 per cent of these isolates were different to those isolated preflushing and 84 per cent of ears had different sensitivity patterns (Hettlich et al, 2005), therefore, isolation of a specific pathogen in rabbits may be misleading.
Up to 30 per cent of rabbits with respiratory pasteurellosis in one study had subclinical otitis media and evaluation of the rabbit for underlying respiratory disease is vital. It is also possible for a rabbit to have a positive culture from the bulla, but be negative on nasal swabs. Other agents cultured from otitis media cases include Bordetella bronchiseptica, Staphylococcus, Escherichia coli and Pseudomonas aeruginosa.
Diagnosis

in rabbits.
Clinical examination may yield clinical signs consistent with otitis externa, with distortion of the base of the vertical canal or purulent material being present. A neurological examination should be performed, particularly assessing the function of cranial VII, VIII and IX nerves (Table 1).
The facial nerve (VII) relays sensory information from, and provides motor activity to, the mandible, maxilla and eyelids. The rabbit should be able to open its mouth, blink and its nose should twitch. A small needle can be used to elicit a twitch of the muscles of the face. Head shaking or moving away from this indicates a central component. The menace and corneal responses are also dependent on facial nerve function.
The vestibulocochlear nerve (VIII) is evaluated by a response to a sudden loud noise or rattling a food bag. Many owners may be able to provide some useful clinical history, but if the disease is unilateral it can be difficult to quantify. The nerve is responsible for balance, and nystagmus may be induced by postural changes. Placing and balancing tests can also be used.
The glossopharyngeal nerve (IX) is best assessed by tongue movement and taste. Enrofloxacin or metronidazole can be dropped on the tongue, which should elicit a response.
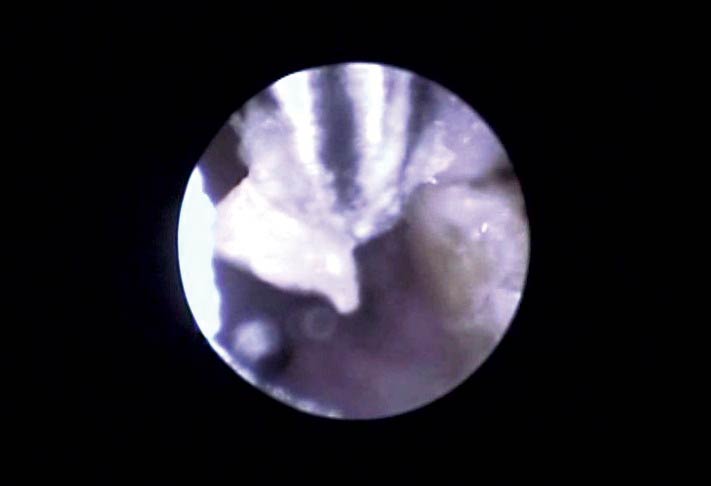
Otoscopic examination (Figure 10) or endoscopic examination of the ear canal under anaesthesia (Figure 11) can help confirm the extent of the infection. For endoscopy, the rabbit can be placed in lateral or ventral recumbancy. Saline insufflation can be used to aid visualisation of the ear canal. It can be difficult to identify the tympanic bulla if there is a large amount of material in the canal and difficult to confirm rupture. Removal of material with cotton buds, flushing or grasping with endoscopic forceps (Figure 12) may help, but runs the risk of mucosal trauma, leading to local bleeding into the canal hindering visualisation further. However, endoscopic biopsies of tissue or exudate deep within the canal can be submitted for culture and histopathology. As the biopsy instrument is sterile until it is passed out the end of the endoscopic biopsy sheath this will yield more reliable results than a culture swab passed down the ear canal, which may pick up more superficial contaminants.
Positive contrast canalography is also a technique to consider. Contrast material may pass into the bulla and confirm a ruptured tympanic membrane. If otitis media without externa is suspected, the tympanic membrane can be evaluated endoscopically and an injection/ aspiration needle used to obtain samples from within the bulla if fluid or purulent material is identified behind the tympanic membrane that may also lead to a bulging of the membrane into the canal.
A myringotomy (opening the tympanic membrane) will allow for this material to pass into the external ear canal (easing pressure and maybe reducing the risk of otitis interna), but a surgical approach to the otitis media is preferred.
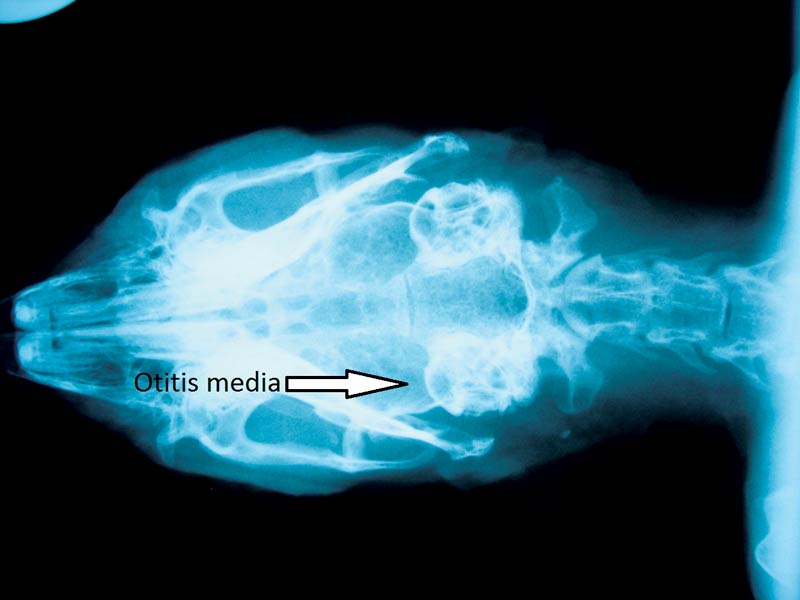
Radiography is commonly performed under anaesthesia or sedation. The dorsoventral view is reported as being the most useful (Figure 13) although lateral, oblique views and open mouth cranial caudal views can also be taken. Oblique views should be between 30 and 70 degrees to visualise the bullae separately. Increased sclerosis of the bone or radio-opacity of the bulla are often reported to be the identifying signs of otitis media. Radiography is a poor diagnostic technique in dogs and many cases of otitis media in rabbits may well be missed. This generally results in a tentative diagnosis at best and many cases are treated medically as a result.
It is impossible to quantify the level of chronic pain present with this condition. The author’s opinion is any cases of otitis media identified on CT examination should be treated, with a view to obtaining a complete resolution. It is unclear if neurological signs will improve or resolve with medical or surgical treatment of otitis media. However, if the otitis media is generated by pressure being applied to the oval or round windows then alleviating the pressure by surgical removal may provide the best option for resolution.Quality of life should be taken into account when considering surgery on those rabbits with marked neurological signs.
- Please note drugs mentioned in this article are used under the cascade.
References
- Deeb B J, DiGiacomo R F, Bernard B L and Silbernagal S M (1990). Pasteurella multocida and Bordetella bronchiseptica infections in rabbits, Journal of Clinical Microbiology 28: 70-75.
- Dickie A M, Doust R, Cromarty L, Johnson V S, Sullivan M and Boyd J S (2003). Comparison of ultrasonography, radiography and a single computed tomography slice for the identification of fluid within the canine tympanic bulla, Research in Veterinary Science 75(3): 209-216.
- Hettlich B F, Boothe H W, Simpson R B, DuBose K A, Boothe D M and Carpenter M (2005). Effect of tympanic cavity evacuation and flushing on microbial isolates during total ear canal ablation with lateral bulla osteotomy in dogs, Journal of the American Veterinary Medical Association 227(5): 748-755.
- Jass A, Matiasek K, Henke J, Kuchenhoff H, Hartmann K and Fischer A (2008). Analysis of cerebrospinal fluid in healthy rabbits and rabbits with clinically suspected encephalitozoonosis, Veterinary Record 162: 618-622.
- Jeklova E, Jekl V, Kovarcik K, Hauptman K, Koudela B, Neumayerova H, Knotek Z and Faldyna M (2010). Usefulness of detection of specific IgM and IgG antibodies for diagnosis of clinical encephalitozoonosis in pet rabbits, Veterinary Parasitology 170: 143-148.
- Keeble E (2011). Encephalitozoonosis in rabbits – what we do and don’t know, In Practice 33: 426-435.
- Keeble E and Shaw D (2006). Seroprevalence of antibodies to Encephalitozoon cuniculi in domestic rabbits in the United Kingdom, Veterinary Record 158: 539-544.
- King A M, Hall J, Cranfield F et al (2007). Anatomy and ultrasonogra-phic appearance of the tympanic bulla and associated structures in the rabbit, Veterinary Journal 173: 512- 521.
- Mayer J (2011). Otology of the rabbit: anatomy, physiology and surgery, Saturday Proceedings of the AEMV Congress, Seattle 47-52.
- Rohleder J J, Jones J C, Duncan R B et al (2006). Comparative performance of radiography and computed tomography in the diagnosis of middle ear disease in 31 dogs, Veterinary Radiology and Ultrasound 47: 45-52.
- Snyder S B, Fox J G and Soave O A (1973). Subclinical otitis media associated with Pasteurella multocida infections in New Zealand white rabbits (Oryctolagus cuniculus), Laboratory Animal Science 23(2): 270-272.
- Smith D T, Webster L T (1925). Epidemiological studies on respiratory infections of the rabbit. VI etiology of otitis media, The Journal of Experimental Medicine 41: 275-283.
Meet the authors
Kevin Eatwell
Job Title