1 Sept 2021
Mónica Guerrero Méndez discusses how to manage this systemic disease in psittacines, birds of prey and waterfowl, using a parrot case example.

Figure 1. The patient, a one-year-old double yellow-headed Amazon parrot.
Lead poisoning has been highly reported in birds. The percentage reported in psittacines is slightly higher than that reported in birds of prey and waterfowl. The lead source in psittacines can be found in the lead putty used for windows, paint, solders, electrical clips and in some avian cages that do not follow certain safety specifications. The lead shot ban, which came into place in England in 1999 prohibiting the use of lead shot and fishing weights, decreased the amount of lead in the environment. Due to this, the incidence of waterfowl suffering from lead poisoning presenting in wildlife hospitals has dramatically reduced over the years.
In birds of prey, lead is normally ingested in the form of gunshot pellets within carcases that have been shot; however, birds of prey have the ability to cast (regurgitate) their meal within 12 to 20 hours post-ingestion, making treatment easier. When feeding raptors, careful feeding practices are paramount.
When caught early – and depending on the toxicity levels found in the body, which can be determined by collecting a blood sample – lead toxicity can be easily managed. Birds require extensive nursing and supportive care, together with chelation therapy and follow-ups once the patient is stable.
Lead poisoning is a systemic, non-infectious disease affecting mostly companion birds such as psittacines and birds of prey. Lead poisoning is less commonly reported in waterfowl since the lead shot ban came into place in 1999, resulting in less lead in the environment.
The prolonged exposure to this heavy metal can cause chronic intoxication, although acute intoxication has also been reported when the ingestion of particles has been large. The lead particles ingested are normally retained in the ventriculus; however, a small amount of the lead absorbed by the gastrointestinal tract can be stored in the bird’s bones and organ tissues, such as the kidneys.
The severity of the signs is determined by the amount of lead ingested, which can be measured in a blood sample collected into lithium heparin tubes. The results range from:
The approach to diagnosing and treating lead poisoning is similar for all avian species; calcium ethylenediamine tetra-acetic acid (EDTA) had been widely used as the main chelation agent in these cases; however, its use has now been discontinued.
Not all veterinarians are able to recognise lead poisoning unless they have come across a case previously. The severity of the signs depends on the amount of lead ingested and the concentration found in the blood. Three clinical presentations of lead poisoning can be found and these can be classified as:
A diagnosis of lead poisoning is generally made based on the clinical signs, although if vomiting is not present, this can be confused with hypocalcaemia; the diagnosis is confirmed by lead blood levels and radiography, in which the lead will appear as extremely radiodense particles.
Calcium EDTA at a dose of 50mg/kg intramuscularly every 12 hours and penicillamine at a dose of 55mg/kg orally every 12 hours had been widely used to treat lead poisoning, but the side effects of calcium EDTA can be severe – especially in mammals – causing nephrotoxicity, depression, vomiting and diarrhoea, and so its use has now been discontinued.
Diazepam is generally used when convulsions and fits are present, and antiemetics can be administered when vomiting is reported. Charcoal can also be used to help reduce lead absorption. Small particles of lead can be passed through the gastrointestinal tract with the aid of laxatives and bigger pieces of lead can be removed surgically or via endoscope once the patient is stable. Patients should be hospitalised and nursed until clinical signs start to disappear.
In this article, the author will discuss the management of acute lead poisoning in a double yellow-headed Amazon parrot, where she took a slightly different approach.
A suspected case of acute lead poisoning was presented to the author as an emergency; according to the owner, the patient had potentially been exposed to lead. The patient (Figure 1), a one-year-old double yellow-headed Amazon parrot (Amazona oratrix) weighing 500g, showed severe neurological impairment. The patient was experiencing seizures, was unable to stand upright and was vomiting convulsively.

Clinical examination at this point was extremely difficult, but obvious paleness of the beak, tongue and mucous membranes of the eyes were reported, suggesting some blood disturbances. A differential diagnosis was made between severe hypocalcaemia and acute lead toxicosis; the second would be confirmed after carrying out relevant diagnostic tests. The patient was admitted for emergency and support care.
Diazepam at 1mg/kg was administered intramuscularly to control the seizures (which seemed to ease fairly quickly) together with maropitant, using it for the first time in parrots at 2mg/kg injection intramuscularly to control the vomiting. As calcium EDTA had been discontinued, the author administered a high dose of calcium salts (calcium gluconate) at 200mg/kg every eight hours.
The parrot appeared very pale on examination of the beak and tongue, so vitamin K at 5mg/kg was administered every 24 hours intramuscularly to help with any internal bleeding or blood disturbances. Amoxycillin/clavulanic acid was also administered intramuscularly at 150mg/kg to prevent any secondary complications. Vitamin B12 was administered at 0.25mg/kg to support the liver; dark green mucous diarrhoea was also reported.
Within an hour post-treatment, the patient had started to show a remarkable improvement, and was kept overnight to be nursed and monitored.
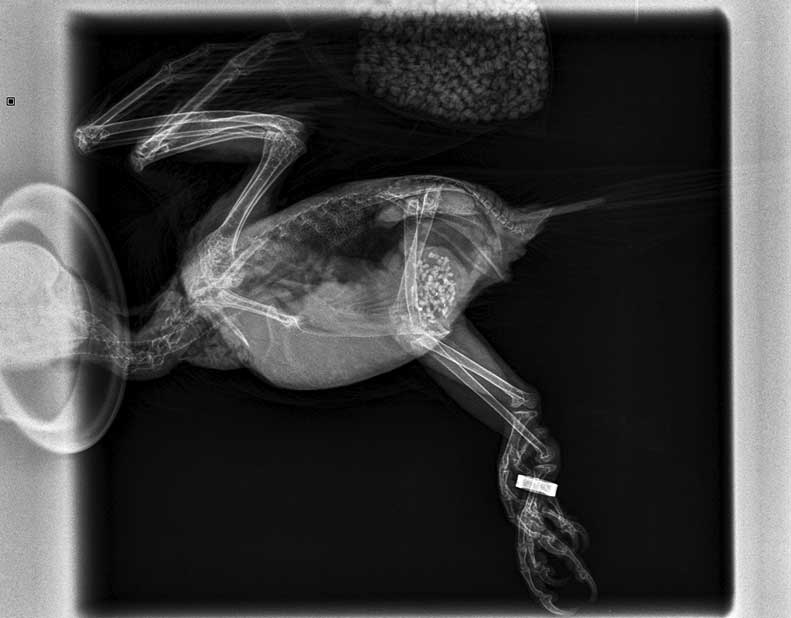
Now that the patient was stable, investigation was carried out. On a lateral and ventrodorsal radiographic examination, some grit in the ventriculus and some small extra radiodense particles (identified as lead fragments) were reported (Figure 2). Venipuncture was then carried out to collect a blood sample for haematology, biochemistry and lead levels, after which prolonged bleeding was reported.
The same treatment protocol continued as for day one, except for the vitamin B12. Since vomiting had stopped, lactulose was added to help shift the lead particles. Penicillamine at 55mg/kg was also added orally, to be continued for three weeks, to prevent absorption of the heavy metal. At this point, the patient was supporting its weight, its balance was stable, the vomiting had stopped, and it had started to eat seeds and fruit. Extra feeds were administered with Harrison’s hand rearing formula.
Biochemistry was unremarkable. In haematology, decreased levels of haemoglobin and PCV were reported, as well as a rise in heterophils (all expected as a response to lead toxicity). The lead levels were 39.1 micrograms/dl, indicating exposure to the heavy metal; clinical signs of lead exposure may not always be obvious, but they were in the author’s patient’s case (Tables 1 and 2).
| Table 1. Sequence of bloods in a double yellow-headed Amazon parrot (Amazona oratrix) being diagnosed with lead poisoning | |||
|---|---|---|---|
| Results | 14/07/2021 | 11/07/2021 | Normal ranges |
| Albumin | 15g/L | 15g/L | (12.0-22.0) |
| Total protein | 38g/L | 40g/L | (33-53) |
| Uric acid | *55umol/L (low) | 65umol/L | (77.7-333) |
| Bile acid | 62umol/L | 64umol/L | – |
| Aspartate aminotransferase | 103IU/L | 105IU/L | (35-200) |
| Creatinine kinase | *0.2IU/L (low) | *0.8IU/L (low) | (64-322) |
| Glutamate dehydrogenase | 9.4u/L | 10u/L | – |
| Calcium | 2.10mmol/L | 2.15mmol/L | (1.87-2.42) |
| Ionised calcium | 0.91mmol/L | 0.95mmol/L | – |
| White cell count | 10.2 109/L | 10.5 109/L | (5.0-17.0) |
| Haemoglobin | *11.9g/dL (low) | 13g/dL | (12.7-16.5) |
| PCV | *36% (low) | 41% | (41-53) |
| Mean corpuscular haemoglobin concentration | 33.1g/dL | 33.5g/dL | – |
| Heterophils | *77% 7.9 109/L (high) | 75% 7.6 109/L | (3.4-7.81) |
| Lymphocytes | 23% 2.35 109/L | 24% 2.4 109/L | (2.2-7.37) |
| Film comment: no polychromasia, but mild hypochromia within erythrocytes series. Mild heterophilia noted, but no toxic or macrophaging leukocytes seen. Thrombocytes appear normal in structure, mildly increased in number and are aggregated in large clumps on film. | |||
| Table 2. General guidelines for lead levels and results for the author’s patient a month apart | ||
|---|---|---|
| Patient’s lead levels 14/7/21 | 39.1ug/dl | Exposure (clinical signs may not be apparent) |
| Patient’s lead levels 11/8/21 | 20ug/dl | Normal levels |
| Laboratory lead levels ranges | <20ug/dl | Normal levels |
| 20-59ug/dl | Exposure (clinical signs may not be apparent) | |
| 60-99ug/dl | Clinical toxicity | |
| >100ug/dl | Acute, severe toxicity | |
As the patient was making very good progress, the same treatment plan continued, except that the calcium gluconate was replaced by an injection of calcium EDTA. This was made up using an EDTA blood tube in which 1ml of saline was added; the mix was then injected intramuscularly once daily for two consecutive days.
The patient was re-radiographed to assess the lead particles and these had already started to shift through the gastrointestinal tract. The patient was then discharged home on lactulose (to be continued for a week); penicillamine (to be continued for three weeks) and amoxycillin/clavulanic acid tablets (to complete a seven-day course; Figures 3 and 4).
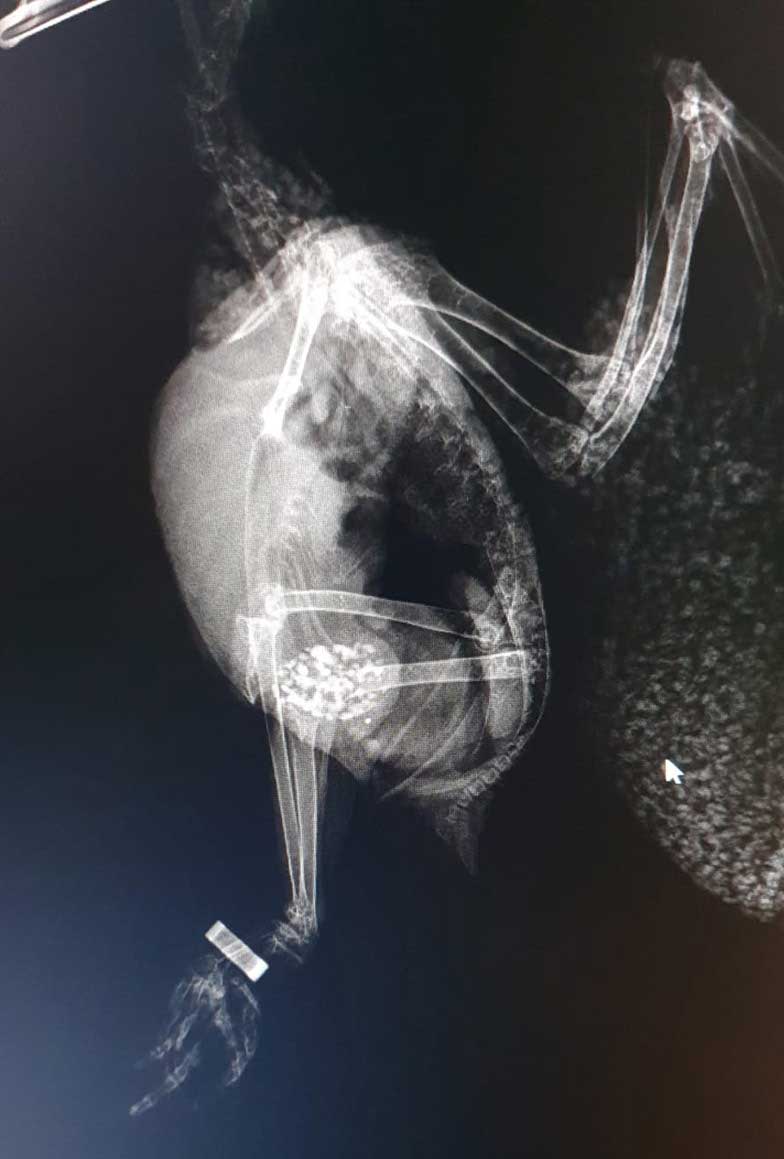
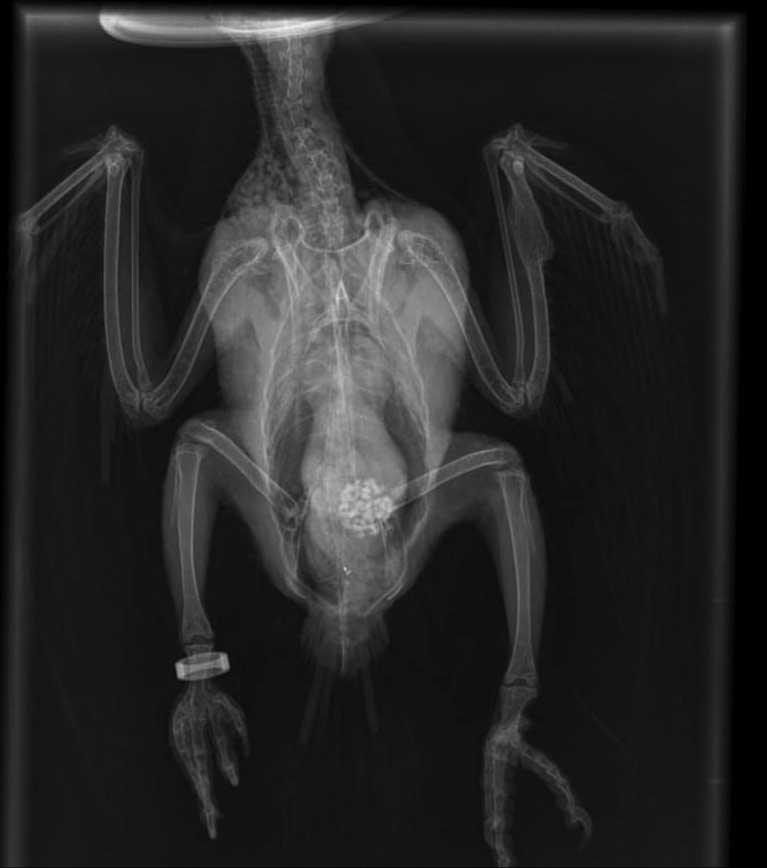
On a follow-up consultation, the mucous membranes, tongue and beak had started to recover pink colouration, and the patient was completely back to normal. The only medication administered at this point was penicillamine.
Recovery continued to be unremarkable. A further blood sample was collected to assess the anaemia, heterophils and lead levels, and all these parameters had returned to within a range that indicated that the lead poisoning had been successfully managed. A follow-up radiograph showed that the lead had successfully shifted alongside the intestinal tract.
Lead poisoning is more commonly reported in companion birds (psittacines and birds of prey) than in waterfowl since the lead shot ban came into place in 1999.
Some of the acute clinical signs of lead poisoning – including incoordination, tremors, fits and neurological signs – can initially be confused with severe hypocalcaemia by the inexperienced clinician. This is why it is important to diagnose the case as soon as possible to be able to start the correct treatment.
Radiograph examinations, together with a full blood test, are the main diagnostic tools in lead poisoning. In this case and on collecting clinical history from the owner, lead poisoning was highly suspected. Paleness of the beak and mucous membranes, blood disturbances, and the watery and mucous green diarrhoea were also helpful in diagnosing the condition. The patient was admitted and support care was started immediately.
Calcium EDTA had been one of the main chelatives used in these circumstances, but having been discontinued, the author decided to administer calcium gluconate at 200mg/kg intramuscularly every eight hours instead. Within an hour, this seemed to have controlled the incoordination and the tremors.
Two days post-calcium gluconate treatment, an injection prepared by mixing 1ml of saline into an EDTA tube was administered intramuscularly and repeated the following day. The author also decided to use maropitant to control the vomiting, which, in her opinion, works faster than metoclopramide.
Lateral and ventrodorsal radiographs, together with a blood sample to assess the lead level ranges, confirmed the diagnosis. The patient was hospitalised for four days until he was stable enough to go home. A remarkable improvement was reported on day 10 post-treatment. Another follow-up appointment on day 28 post-treatment was scheduled to repeat the full bloods and the results were unremarkable; the lead levels in the bloodstream had now decreased to normal levels.
The results achieved in this case, using a slightly different approach drugs-wise, were excellent and perhaps could be extrapolated to future cases; the advantage of using calcium gluconate is that there are fewer side effects.
The author acknowledges South Essex Wildlife Hospital, Orsett Road, RM16 3BH and all the members of staff, including her assisting nurse Kate Theobald for nursing her patient, and for their compliance.
The author also acknowledges Ross Brown – professional proofreader, business writer and editor – for kindly proofreading this article.
Drugs in this article are used under the cascade.