23 Nov 2015
Managing tortoise shell injuries
Kevin Eatwell reviews the steps to be taken in stabilising shell injuries in tortoises and then moves on to the methods used in repair to ensure successful outcomes.
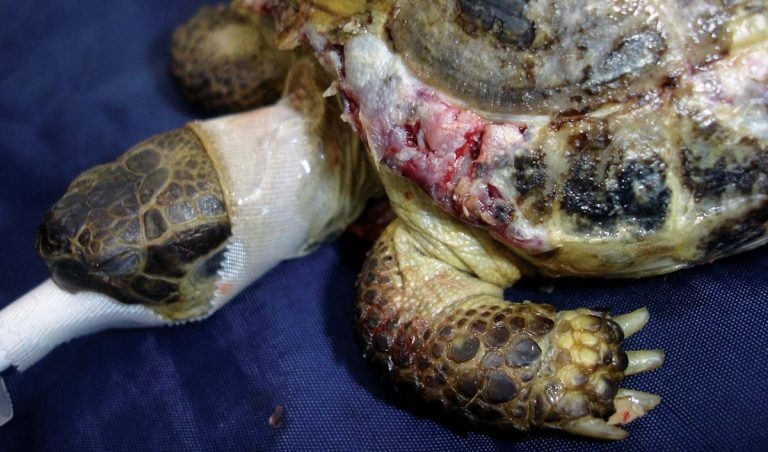
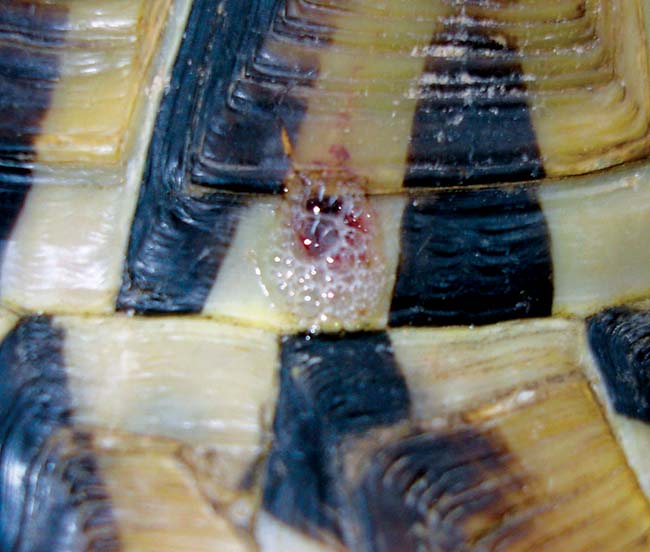
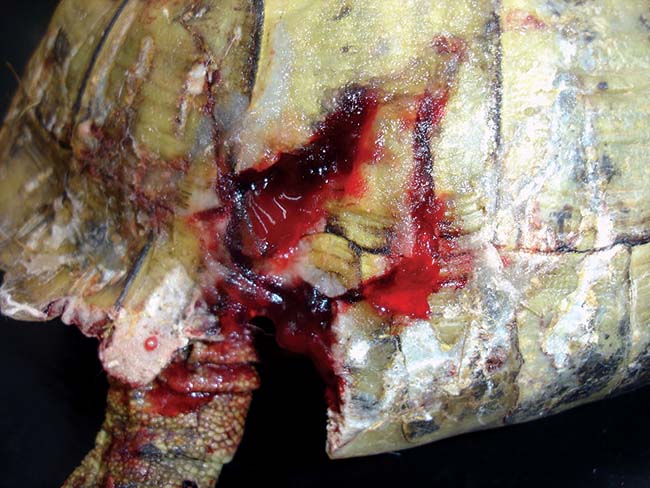
Figure 3. Most shell injuries consist of gnawing and lead to damage to the keratin scutes and the dermal bone, which can be superficial.
Shell injury is one of the most common reasons for a tortoise to be presented to a veterinary practice. Many tortoises in the UK are housed outside in gardens for some or all of the time and this exposes them to a range of potential injuries.
When dealing with an injured tortoise it is important to understand shell lesions need no different approach to how you would work with skin wounds or fractures in mammals and the same basic principles apply.
However, reptiles as patients do need different management and you need to have a degree of understanding of a tortoise’s altered anatomy and physiology. This is critical to ensure a successful outcome of the case.
The chelonian shell is a biologically active modification of both the epidermis and dermis, and consists of an upper carapace and a lower plastron connected laterally by bone bridges. The shell has evolved from the rib cage, vertebrae, thoracic and pelvic girdles. It consists of dermal bone plates and is covered by epidermal keratin scutes.
These do not directly overlie the bone plates. All the tissue is capable of regeneration in the event of injury. Although the epidermal scutes are essentially horn or nail, the dermal bone is highly sensitive tissue. Thus, tortoises with shell fractures or damage will be in a considerable amount of pain.
New epidermal scutes are produced with each growth period and are retained for an extended time period before being lost. Spur-thighed tortoises have a flexible plastron hinge, which should be preserved.
Initial consultation and assessment
Shell trauma is a common presentation in chelonians and can be as a result of injury from lawnmowers, strimmers, being dropped, dog bites, rodent trauma, thermal burns and fires, blunt trauma from other tortoises or due to shell infections. This can mean there are areas of infection, devitalised bone or fractures. Surgical intervention is required in many cases, including shell repair.
With any reptile consultation an extended clinical history is required so husbandry can be reviewed. Healing can take a long time in tortoises and immediate concerns should focus on the tortoise having the right environment to allow it to heal. A tortoise kept too cold or one due to enter hibernation before healing has taken place will not recover as expected. There may need to be a discussion with the owner regarding how to keep a hibernating species awake over the first winter.
A chelonian presenting with shell injury requires a thorough examination as underlying structures can be damaged, depending on the location of the injuries. Areas of potential damage include the spine, lung fields or the coelomic cavity. The animal’s mobility should be assessed and the limbs, cloaca and head examined for signs of trauma or neurological deficits. Puncture wounds entering the coelomic cavity or the lung fields should be noted and may require further evaluation. If the lung fields are punctured, typically, a frothy bloody or serous discharge will be evident as air escapes via the hole (Figure 1).
Obviously, flushing these wounds is contraindicated. As tortoises have no diaphragm and respiration is accomplished by contraction of smooth muscle within the lung and skeletal muscles of the limbs, it is not necessary to create a negative pressure within the lung fields. As a result, respiration can continue normally despite the breach.
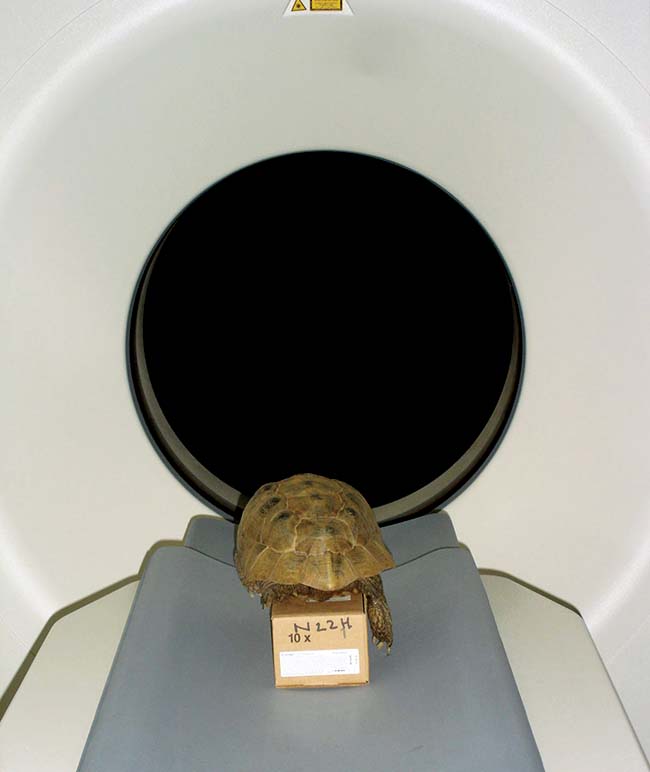
Radiography and, ideally, CT examination may be required for the full evaluation of orthopaedic injuries (Figure 2). Radiography is often a poor option for these as it is difficult to distinguish between the differing bone layers due to superimposition. CT is far superior.
Most shell injuries consist of gnawing and lead to damage to the keratin scutes and the dermal bone, which can be superficial (Figure 3). Anaesthesia is likely to be required to facilitate thorough assessment and management of wounds. Contaminated wounds should be gently flushed (unless the lung fields are entered) and grossly debrided in the first instance.
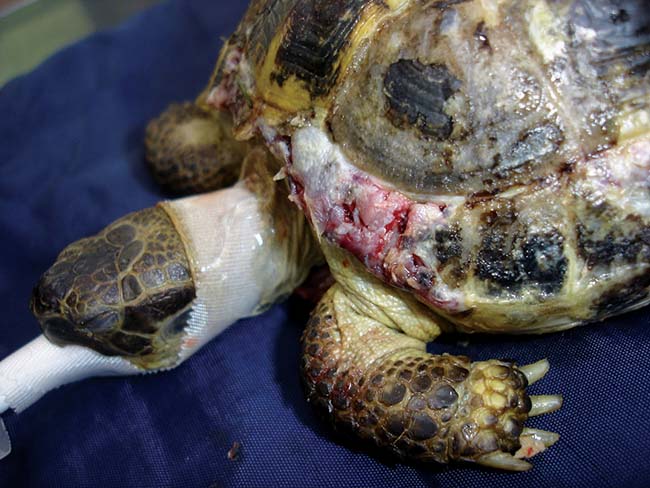
Fractures are usually compression fractures (Figure 4) and these will require either removal (if devitalised) or lifting back into place, and surgical repair under anaesthesia. In most cases, underlying coelomic structures may just be compressed. In other cases there may be significant bone deficit. This is of no real concern around the head and legs (but the deficit will remain). If over the plastron or carapace then time will be needed for new bone to regenerate.
Initial treatment and stabilisation
Subsequent infection of these injuries is likely. Gram-negative aerobes are obviously a common finding from reptile cultures, but many of these injuries will be contaminated with soil or bacteria from the teeth of the biting animal. As a result, anaerobic bacteria may also be a problem.
Although taking a culture result would be wise, presumptive therapy that covers likely opportunistic pathogens is wise. Ceftazidime (20mg/kg every 72 hours) or ticarcillin (100mg/kg every 48 hours) are suitable choices, as other antibiotics such as enrofloxacin are not effective against anaerobes. Antibiotics would not be indicated for acute clean injuries, which can simply be covered.
Analgesia is of critical importance in these cases. Meloxicam doses used range from 0.2mg/kg to 0.4mg/kg every 24 hours. Opioids should also be considered and morphine (1mg/kg to 4mg/kg) is considered to be the most suitable for these cases.
Other opioids, such as butorphanol or buprenorphine, may have questionable benefits.
Topical treatment of shell wounds focuses around the same general principles of wound care. To heal, a wound must be free of devitalised tissue, have a good blood supply, be clear from infection and have a moist environment.
The initial aim is to facilitate debridement, control infection and encourage fluid ingress into the wound. Debridement may be less of an issue given the regenerative process occurs from deeper layers and necrotic material is simply pushed off over the passage of time (years). This tissue can act as a useful dressing to prevent bacterial ingress and cover the wound; conversely, removal should aid granulation and new bone growth.
Although the use of iodine or chlorhexidine topically is common in reptiles, this causes problems as it damages tissue, impairing the healing process and has minimal effect on the bacterial load. Other common agents used include topical ointments or creams, such as silver sulfadiazine. Most agents are irritating and can impair healing due to being cytotoxic or lead to a foreign body reaction in the tissue.
In an emergency situation the author would consider gentle irrigation and removal of obvious debris, then using a hydrogel and bandaging the wound while the patient is being stabilised. More thorough debridement will require anaesthesia.
Irrigation can be with saline at the clinic, but tap water will suffice if the wound is heavily contaminated and you are giving telephone advice to the owner. Medical grade Manuka honey is another option as it has many of the same beneficial effects as the hydrogels, encouraging wound debridement, facilitating fluid ingress and having antibacterial effects.
The importance of keeping acute wounds moist should not be forgotten. Covering the wound allows for increased leukocyte migration into the moist environment, facilitating infection control and vascularisation.
Dressings used at this stage should be aimed at keeping the moist environment (but not allowing excessive moisture build-up), protecting the wound from further damage or ingress of foreign material, reducing bacterial invasion, facilitating debridement and not leading to a tissue reaction (for example, cotton wool fibres entering a wound when this is being used to clean it).
Suitable dressings to consider include foam-based dressings that can also have an adhesive backing. Examples include such products as Allevyn (Figure 5). Hydrogels can be used to pack deficits in wounds. For wounds with large deficits the use of negative pressure therapy may be advantageous.

Fluid therapy will be required depending on the anticipated fluid loss from the wound. Bathing may also be contraindicated by the nature of the wounds and so providing maintenance at least is sensible. Fluid therapy rates used in reptiles are between 20ml/kg to 30ml/kg per day.
The tortoise also needs to be placed into the correct thermal environment to enable the healing process and drug assimilation.
Anaesthesia, surgical debridement and repair
Anaesthesia and surgical debridement or repair can be performed once the tortoise has been stabilised.
The author would consider using morphine and a low dose of ketamine as a premedicant for these cases. Ketamine has largely fallen out of favour as an anaesthetic agent in reptiles, but given it has significant analgesic properties in other species, the author has embraced it again for these potential benefits. A low dose of 20mg/kg to 30mg/kg is used. Induction is then typically via the jugular vein using alfaxalone (10mg/kg to 15mg/kg) or propofol (10mg/kg).
The trachea bifurcates into the two bronchi very quickly and the glottis is usually closed unless the tortoise is taking a breath. This makes intubation hard and often custom-made endotracheal tubes will be required.
Once intubated, intermittent positive pressure ventilation will be required. Respiration is largely facilitated by the movement of the front legs and so the tortoise should be positioned, if possible, to optimise this.
Temperature control is critical and an oesophageal or cloacal temperature probe should be considered and a variety of techniques used to maintain core temperature. The author usually aims to increase room temperature to the upper end of the surgeon’s thermal tolerance (24ºC to 26ºC) and use heat mats below the patient. Having a warm vivarium ready for recovery is important.
Once the patient is anaesthetised the sites can be surgically prepared. This is often with dilute iodine, but given the risk of tissue damage this should be rinsed off with sterile saline once it has had sufficient contact time (two minutes) with the surgical field. The author would also consider keeping scrubbing of open wounds minimal, focusing more around the wound edges and leaving wound cleaning to surgical debridement and intraoperative wound irrigation, particularly as debris deactivates iodine.
Sterile cotton buds, gauze swabs and irrigation with saline can be used to facilitate initial debridement. All loose bone fragments should be removed.
All aspects of the wound should be explored to evaluate for underlying damage to tissues. If there is sufficient space and a deep pocket is identified, endoscopic assessment may be indicated. Deep cultures can be taken from tissues with a swab or a bone biopsy may be indicated at this stage. It is best to take cultures before starting antibiotic therapy so if you are planning to take one, delay antibiotics until after the sample has been taken and avoid flooding the tissues with an iodine preparation.
Bone rongeurs and Volkmann spoons are useful to facilitate debridement of lesions and remove areas of grossly contaminated or devitalised bone. All surfaces should be debrided back until wound edges are clean and the surface is pink and bleeding. In some cases, diamond discs may be required to create a clean edge and open out deeper wounds.
Debridement of thermal injuries should be delayed as it can be difficult to identify tissue viability in the early stages. Debridement under anaesthesia will be required in many cases, but it is best to treat medically until the full extent of thermal damage can be appreciated. Thermal injuries can present as exudative areas of the shell (typically carapace from heat lamps or fires and plastron if the tortoise has been on a heat mat). Obvious charring may be evident in extreme cases. Immediate attention to analgesia, fluid therapy and antibiosis is required.
Having debrided the wound consideration must next be given to closure. In some cases where there is a compression fracture this can be elevated back into place and fixed. Other cracks may require compression to be applied laterally to hold them in place. As these fractures are on the surface a wide variety of methods can be considered.
The most important point is to understand these are healing wounds that have the potential to develop infection or may need further debridement. Close monitoring of wound progression may be needed and the method of repair should facilitate this.
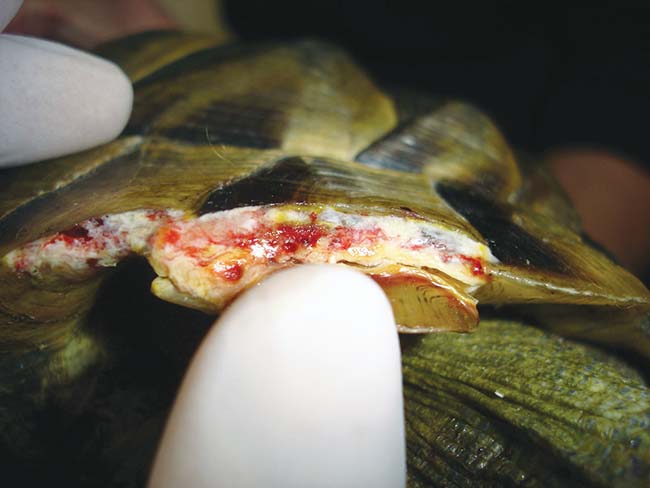
Covering wounds with poly(methyl methacrylate), fibreglass and epoxy resin or dental acrylic is generally contraindicated. However, an acute clean wound (Figure 6) can be packed with a hydrogel (to prevent ingress of the repair material into the wound, which would significantly impair healing) held together (either manually or using Steri-strips, for example) and then the repair material spread over the surface.
Very rarely, surgical implants may be required. These would typically be used in wounds that would be left open, as the use of implants risks failure or increased infection around the sites of fixation. Many liquids used are exothermic and care must be given to avoid thermal damage of the wound itself.
Surgical repair of sites can be performed with a wide variety of implants. No specialist implants are needed. Thought has to be given to maintaining the shell shape and also to ambulation. Implants may interfere with gait and lead to limb or head trauma.
Bone plating still has its place in the repair of wounds. Typical sites for a bone plate will be on the central plastron or towards the more mobile femoral or pectoral scutes. The plate provides a rigid longitudinal fixation, which no other method will provide, if there is complete loss of bony union in a distal fragment. Care has to be taken when selecting screw length to prevent trauma to underlying soft tissues. There is also good visibility around the implant to check the healing process (Figure 7).

Screws and IMEX external fixator pins can be used to act as anchor points in bone fragments and then orthopaedic wire or cable ties can be used as a tension band to appose the bone.
Techniques using dental acrylic or poly(methyl methacrylate) to fix hooks or blocks on the shell to act as anchor points have been used as a low-cost way of stabilising fractures.
Alternatively, screws and external fixator pins can be used to fix a small lateral fragment in place.
Orthopaedic wire can be placed as a hemicerclage wire to hold a fragment in place via two pre-drilled holes (Figure 8). Implant removal can be performed after 12 months and any holes filled with intrasite gel and covered with either an adhesive dressing (which can be regularly replaced while the wound is healing and allow assessment) or Technovit, for example.
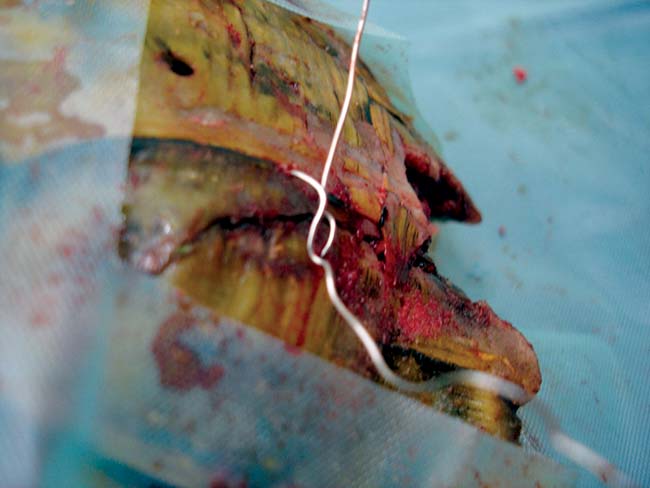
These techniques allow wound irrigation where infection and contamination may progress unchecked.
However, if deeper irrigation is required then an irrigating catheter can be placed. This can be used for ongoing flushing, antibiotic therapy and analgesia.
A small drill hole is made and a 24-gauge catheter (cut short) is placed and secured with poly(methyl methacrylate) or dental acrylic. The catheter is removed at a later date and the hole can be covered.
Recovery and wound healing
Dressings can be applied around the implants after surgery if there are significant deficits present (Figure 9). Bandage changes may need to be daily in the initial period after repair, but it is probable the tortoise will remain as an inpatient for further analgesia and evaluation.
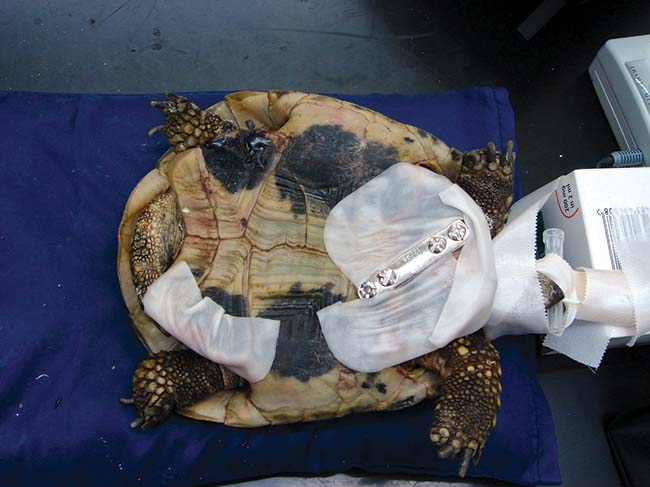
Once ready to go home, bandaging is generally every 72 hours to coincide with further antibiotic dosing with ceftazidime.
Generally by about 12 weeks after surgery a firm granulation bed should be present in any deficits, depending on the temperature of the patient. Regeneration is from the soft dermal tissues membrane and not from the wound edges, thus the size of the wound has no bearing on its rate of healing. This granulation tissue then ossifies.
Ossification and development of full thickness dermal bone can take one or two years. However, once the granulation bed has been formed the region is resistant to infection and the wound simply requires covering to prevent further trauma. X-ray film or another material can be used to cover the area secured with tape.
- Please note drugs mentioned are used under the cascade and not licensed for use in reptiles in the UK.
