11 Nov 2025
Elisabetta Mancinelli DVM, CertZooMed, DipECZM, MRCVS and Molly Rogerson RVN use the specific example of a dental procedure in rabbits to emphasise the importance of welfare at every stage when dealing with an often-fragile patient group.
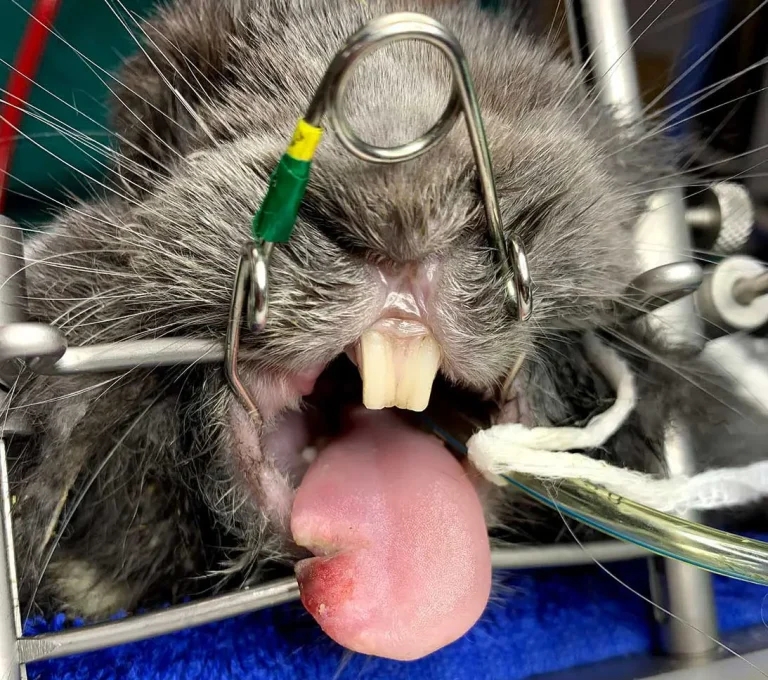
Figure 1. This image shows a rabbit anaesthetised and intubated to undergo an oral examination and dental procedure. A large laceration is visible on the right lateral aspect of the tongue, associated with dental abnormalities that were identified on diagnostic imaging and which will be addressed during this procedure.
Veterinary surgeons have a professional and ethical duty to prioritise the health and welfare of all patients. Rabbits present unique challenges in clinical practice due to their peculiar anatomical, physiological and behavioural characteristics.
Due to their status as prey animals, rabbits often mask signs of illness and respond poorly to stressors, often resulting in delayed presentation and advanced disease at the time of clinical intervention.
Therefore, it is essential to remember that welfare begins from the moment of entry into the clinical environment and continues through diagnosis, treatment and discharge. The lecture will explore a comprehensive approach to improving surgical outcomes in these species.
Using the specific example of a dental procedure, emphasis will be placed on preoperative optimisation, anaesthetic and analgesic protocols tailored to rabbits, intraoperative considerations, and postoperative care strategies to reduce morbidity and promote recovery, ensuring not only survival, but a return to a good quality of life.
The session is ideal for general practitioners, exotics clinicians and veterinary nurses aiming to enhance their care of this often-fragile patient group.
Rabbits demonstrate profound physiological responses to stress via the hypothalamic-pituitary-adrenal (HPA) axis. Stress triggers corticotropin-releasing factor (CRF) release, leading to glucocorticoid production (Smith and Vale, 2006). While adaptive short-term, prolonged exposure can result in immunosuppression, hepatic lipidosis, cardiovascular compromise and gastrointestinal (GI) stasis (Goldkuhl et al, 2010; Varga, 2014).
Rabbits are considered catecholamine-driven animals, very prone to stress and with a high metabolic demand. If handled too roughly, stress-induced catecholamine, and endogenous corticosteroid release, can result in tachycardia, hypertension, reduced renal perfusion and hyperglycaemia. If a rabbit is already ill, this process can worsen dramatically its clinical conditions, resulting, in extreme cases, in cardiac failure and death due to stimulation of the sympathetic nervous system. Therefore, attempts must be made to reduce stress from first visit to discharge.
Appointment scheduling, environmental control (for example, separating prey from predator species) and reduced waiting times can substantially lower stress levels.
Carriers should be covered, and pre-prepared consult rooms allow efficient and minimal handling. Pre-emptive analgesia may reduce postoperative stress and enhance recovery outcomes (Page et al, 1998). Adequate handling is also essential to avoid serious injury, including spinal trauma.
Compensatory mechanisms are blunted during general anaesthesia, potentially exacerbating underlying hypotension and poor peripheral perfusion. A comprehensive patient assessment is essential and includes a full clinical history, physical examination with oral inspection and laboratory diagnostics, if feasible. Baseline parameters – temperature, heart rate, respiratory rate, mucous membranes and hydration – should be assessed and recorded.
Thoracic auscultation, abdominal palpation and auscultation are also of utmost importance for evaluation of the clinical condition of the patient presented. A visual assessment of body condition score should be made and an accurate bodyweight should be taken. Pre-anaesthetic blood work may assist in identifying subclinical disease. This will allow the clinician to have baseline parameters specific to the patient that can be used throughout the procedure for monitoring and during the recovery phase.
Factors, such as the presence of underlying disease, lack of confidence and expertise of staff involved, anatomical and physiological differences compared to more common species, inappropriate extrapolation of drug dosages, inadequate pain relief, suboptimal patient monitoring and postanaesthetic care are all factors that can complicate a procedure significantly. Ultimately, the principles of anaesthesia are not different compared to those used in dogs and cats and the availability of more advanced equipment greatly enhances the chances of positive results, making the whole experience less stressful for vets and patients.
Rabbits have high metabolic rates and small glycogen reserves, therefore prolonged fasting (for example, prior to an anaesthetic) or anorexia may predispose them to develop hypoglycaemia. Overnight fasting prior to anaesthesia is not recommended in rabbits because of their rapid gastrointestinal (GI) transit time and potential for GI stasis.
Fasting has been advocated to reduce stomach and caecal volumes, which can theoretically compress a rabbit’s small thoracic cavity. However, fasting generally does not change GI volumes enough to be clinically significant. Fasting also exhausts glycogen reserves, resulting in hypoglycaemia and contributing to perianaesthetic ileus. Further to this, rabbits cannot vomit because of their well-developed cardiac sphincter.
In general, recommended fasting times are comprised between zero and four hours maximum, carefully weighing the risks versus the benefits in specific cases. For example, scheduling procedures early during the day may reduce fasting times and elevating the head compared to the abdomen during a procedure may reduce risks of aspiration or reduce compression over the chest.
Hospitalised rabbits should be housed away from visual and auditory cues of predators. Cages should include newspaper and hay or straw bedding, a hide box and a litter tray. Consideration should be given to temperature regulation (17°C to 22°C). Bonded companions may reduce hospitalisation stress, help with recovery time and prevent fighting when the rabbit is reintroduced in the home environment. Dietary consistency should be maintained.
Always have a selection of common rabbit food in the hospital to avoid changing diet while the animal is hospitalised. Alternatively, owners can supply familiar food. Provide a bowl and bottle for water supply. Many rabbits will prefer and will appear to drink more if given water in a bowl (Tschudin et al, 2011). Daily enrichment and monitored exercise can also support recovery and gut motility.
Many rabbits are admitted to the hospital to receive medical treatment or to undergo surgical or other procedures. The literature reports that the overall perianaesthetic mortality rate of healthy pet rabbits is relatively high (1.39% [95% CI 1.14% to 1.64%]) within 48 hours of a procedure (Broadbelt et al, 2008). These values are 5 to 10 times higher than those found in dogs and cats. Nevertheless, with careful planning, these numbers can be dramatically reduced.
Try to plan any anaesthetic early during the day to reduce stress and allow plenty of time for the rabbit to recover from an anaesthetic and to be monitored. Anaesthetic preparation should include having any equipment needed for any planned procedure ready to reduce handling time and stress. Calculate the doses for any anaesthetic and emergency drug in advance. Unhealthy animals should be stabilised, where possible, before undertaking an anaesthetic procedure (for example, fluid therapy started to correct dehydration, assisted feeding commenced in anorectic patients, antibiotic administered if required and pain relief provided). Establishing IV access for fluids and drug administration is crucial in many cases, moreover in an emergency, and it is always recommended when hospitalising a rabbit for any anaesthetic event.
The concept of balanced anaesthesia can be applied to rabbits as well. It is advisable to use multiple drugs at lower overall doses to minimise the adverse effects of each drug, while still providing appropriate anaesthesia and analgesia for the procedure.
This approach may reduce the magnitude of cardiovascular depression under anaesthesia and promote haemodynamic stability. For example, a sedative/analgesic combination may be given during the premedication phase to reduce the stress associated with handling. This will also facilitate administration of an induction agent to allow intubation and delivery of a volatile agent, which can then be used for maintenance. Availability of reversal agents further adds to these benefits.
Classes of drugs commonly employed include sedatives (for example, midazolam, alpha-2 agonists), opioids (for example,, butorphanol, buprenorphine, fentanyl and methadone) and dissociative agents (for instance, ketamine).
Inhalant agents alone, either administered via face mask or induction chamber, are not recommended in rabbits because this method is too stressful, patients can breath-hold (leading to hypoxia, hypercapnia and bradycardia) and struggle, potentially injuring themselves.
The choice of the most appropriate anaesthetic protocol will largely depend on personal preference and familiarity with each of the drugs, the health status and temperament of the patient, the procedure to be performed and the expected levels of pain.
However, even with balanced anaesthesia protocols, morbidity and mortality increase with increased duration of anaesthesia. That’s why it is of utmost importance to prepare the patients, all the drugs, the equipment in advance and provide close patient monitoring so that any problem is promptly identified and corrected immediately.
The Ayre’s T-piece is commonly used for its low resistance and little dead space. However, other non-rebreathing systems (Bain, Mapleson, Magill, Jackson-Rees, Normal elbow) are available. Pre-oxygenation is mandatory to reduce the risks of hypoxia (especially during induction and intubation). Orotracheal intubation, while technically difficult, should be attempted where possible.
Intraoperative monitoring involves continuous assessment of heart rate, respiratory rate, blood pressure (if feasible) and temperature. Tools such as capnography, ECG and Doppler enhance outcomes. Hypothermia is a frequent and preventable cause of anaesthetic morbidity. Hypothermia prolongs recovery, increases potency of volatile anaesthetics, depresses cardiovascular function, causes coagulation disturbances and increases the risks of anaesthetic death.
Intraoral dental procedures are frequently performed in rabbits (Figure 1). Diagnostic imaging (for example, skull radiography, or CT) informs treatment decisions. Availability of suitable dentistry equipment is essential for both diagnosis and treatment (Figure 2). The general guidelines of intraoral dental procedures consist of reduction of abnormal tooth length of clinical crowns, restoration of the occlusal plane to as near normal as possible, and extraction of diseased teeth (Capello, 2016).
Complications and secondary diseases, such as periapical infections, osteomyelitis and odontogenic abscesses, are treated with combined dental procedures and extraoral surgery. Medical therapy (analgesia, antimicrobials or assisted feeding) is essential adjunctively. Avoiding repeated anaesthetics is desirable and, therefore, diagnosis and treatment are ideally often combined.

Postoperative monitoring is critical, as most deaths occur within three hours post-general anaesthesia (Broadbelt et al, 2008). Respiratory depression can continue into the post-general anaesthesia period, so supplemental oxygen should be continued. Rabbits should be housed in a quiet, warm, oxygenated environment. Once the animal is alert and moving around, handling and monitoring may be reduced to avoid unnecessary stress.
Pain assessment is complex in rabbits and must take into consideration the different types and sources of pain. It is important to become familiar with the normal behaviour of rabbits, as well as that of the individual. Balanced analgesia implies the combination of drugs acting at different levels of the pain pathway and it may be more effective and less toxic than one drug given alone.
Opioids, which mainly act at CNS level, and NSAIDs, which mainly act peripherally to decrease inflammation and peripheral sensitisation, may be used in combination. Pre-emptive analgesia can be obtained by administering opiates, NSAIDs and local anaesthetics before a painful procedure to block noxious sensory stimuli from onward transmission to the CNS, thus reducing the overall potential for pain and inflammation.
All emergency drugs should be prepared in advance. Equipment such as oxygen, IV catheters and intubation tools should be ready. Cardiopulmonary resuscitation (CPR) principles apply similarly to other species, using the ABC approach. Success depends on rapid intervention, oxygenation, and circulatory support (Gladden and Lennox, 2019).
At discharge, owners should receive a clear explanation of diagnostic findings, procedures, medication instructions, and supportive care needs. Demonstrations of syringe feeding and administration techniques should be provided. Written discharge instructions and emergency contact details support owner confidence and improve compliance.
Referral should be considered when the required expertise or equipment is not available. Early referral ensures better patient outcomes and should be viewed as a commitment to optimal care, not a clinical failure.
Effective and optimised rabbit anaesthesia requires species-specific planning and execution. Minimising stress, selecting appropriate protocols, monitoring diligently and communicating effectively with owners are critical components of success. By optimising each phase of care – from admission through recovery – we improve outcomes and uphold the highest standards of animal welfare.