11 Sept 2017
Elisabetta Mancinelli discusses one of the most important diseases among females of all species, including humans, in a three-part series.
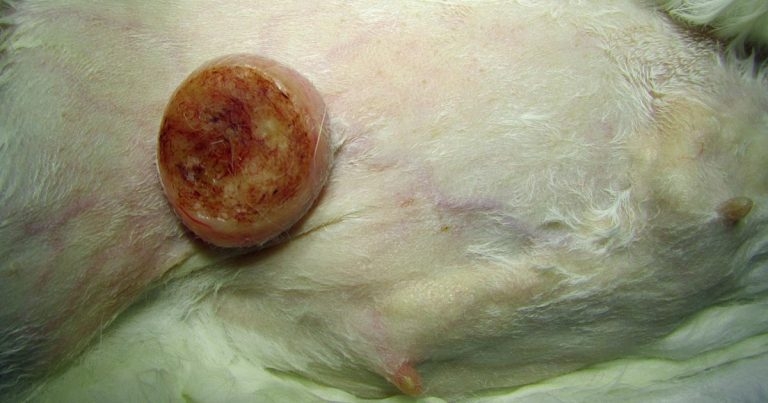
Figure 1. Mammary neoplasia are frequently reported in female rabbits.
Spontaneous mammary tumours are one of the most important diseases among females of all species, including humans.
These tumours are described in many domestic and laboratory species, but their morphology and biological behaviour vary between each of them, and interspecies comparative studies are considered important as they may contribute significantly to the understanding of human breast cancer. Furthermore, as the standards of care of pet small mammals improve and their age increases, many of these studies also represent an invaluable tool for vets seeing rabbits and rodents in daily practice as they may improve knowledge and provide clinically applicable information.
This, the first in a three-part article considering latest research on spontaneous mammary tumours, will focus on rabbits and guinea pigs. The second part will look at rats, while the third, will focus on hamsters and hedgehogs.
Mammary tumours are considered common in pet rabbits (Figure 1), comprising about 20% of all submissions from pet rabbits in one laboratory facility (unpublished data: Baum and Hewicker-Trautwein, 2015). However, data from pet rabbits is lacking as the majority of information available is derived from laboratory or meat animals. Therefore, in their 2015 study, Baum and Hewicker-Trautwein aimed at characterising clinical and histopathological features of mammary tumours (119 tumours in total) in a pet rabbit population. Biopsy samples from 109 rabbits were, therefore, revised. Gender, age, size of tumour and identity of affected mammary glands were evaluated. Age of onset and size of the tumour for carcinomas and benign lesions were compared and tumour morphology was assessed microscopically for histological pattern, cytological appearance, tumour growth pattern and inflammation. A scoring system was also developed for semi-quantitative morphological analysis.
Diagnosis was made according to the World Health Organization (WHO) histological classification for mammary tumours in cats and dogs (Misdorp et al, 1999) applying the current modification for canine mammary tumours (Goldschmidt et al, 2011).
Rabbits were aged 2 to 14 years (mean 5.5 years) and the mean age for animals with carcinomas was significantly higher than for those with adenomas (P <0.05). Only 90 rabbits were of known gender and were all females. The study showed – differently from dogs, in which the caudal mammary glands are more frequently affected – in rabbits the cranial and caudal mammary glands were almost equally affected for carcinomas, while the low number of benign lesions did not allow statistical evaluation. The left and right mammary chains were also equally showing neoplastic changes. The different posture and motion in the two species might explain these findings.
The dominant tumour type was the simple neoplasm. Tubular, cystic-papillary and combined forms comprised about half of the carcinomas and the majority of adenomas. The remaining masses were classified as adenomas or non-neoplastic lesions (for example, duct ectasia). The majority of tumours (88%) exhibited histological signs of malignancy (for example, infiltrative growth, incomplete differentiation and/or cellular dysplasia). This, coupled with the rare occurrence of complex tumour types, is in contrast with what is reported in dogs and more similar to the situation of cats (Misdorp et al, 1972). However, unlike cats, rabbits more often presented with carcinomas with squamous differentiation, commonly combined with strongly malignant morphology and severe inflammation. The actual incidence of metastasis remains to be determined in further investigations.
In another study, Suárez-Bonnet et al (2010) reported different results for guinea pigs. Males were more commonly affected and benign neoplasms were overrepresented compared to rabbits. Benign lesions were mostly tumours (12%), with only one case of non-neoplastic duct ectasia. However, the authors noticed benign processes were smaller and occurred earlier in life than carcinomas, which might indicate transition from benign to malignant lesions with time, as proposed in other species where mammary tumours might arise from hyperplastic lesions.
Greene and Strauss (1949) reported uterine adenocarcinoma was frequently combined with mammary hyperplasia in rabbits, while Greene (1939) stated hyperplastic and neoplastic lesions of the mammary glands were almost always associated with uterine adenocarcinoma in rabbits. More recent studies reported similar results; in a study by Saito et al (2002), 15 of 47 rabbits with adenocarcinoma also had mammary disorders.
In another study (Walter et al, 2010), 4 out of 59 rabbits (7%) with uterine endometrial hyperplasia or carcinoma were found to have mammary adenocarcinoma. A possible hormonal influence could not be ruled out or confirmed in the present study. However, three patients presented uterine neoplasia or dysplasia concurrently with a mammary tumour. Greene and Strauss (1949) also described a marked difference between English and Belgian breeds of rabbit, with regard to the incidence of mammary tumours before the age of three years. The genetic component of this predisposition is, however, difficult to assess in pet rabbits. The majority of tumours were found in “dwarf” rabbits, but this is a poorly defined genetic background. Other more homogeneous breeds were too low in number for statistical evaluation.
The literature is extensive regarding the prevalence of different tumour types in guinea pigs. Spontaneous tumours are considered relatively uncommon in this species, especially in animals under three years of age, but the majority of data available is extrapolated from laboratory settings.
Retrospective reports have described the epidemiologic characteristics, gross morphology, and microscopic features of both benign and malignant mammary tumours in guinea pigs (Andrews, 1976; Blumenthal and Rogers, 1967; Kitchen et al, 1975). According to available literature, mammary tumours are generally locally invasive and rarely metastasise. They are often large and highly vascularised, and their prevalence in male guinea pigs is higher than in other species.
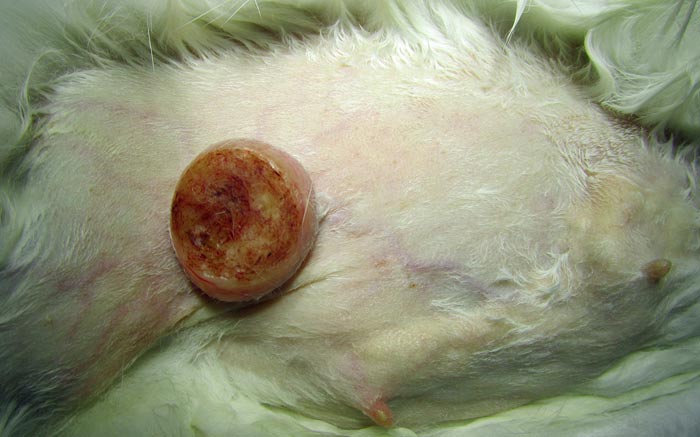
Surgical excision of a primary mammary tumour is possible, but recurrence is likely if excision is incomplete (Andrews, 1976). Suárez-Bonnet et al (2010) designed a study to collect further information on the morphological and immunohistochemical characteristics of spontaneous mammary gland tumours in guinea pigs. Ten mammary gland tumour specimens obtained either from mastectomies or necropsies were analysed. Results showed 87.5% of tumours examined involved animals older than three years of age and two out of 10 occurred in males. This confirmed existing data regarding the higher prevalence in males of this species compared to others (Saba et al, 2007; Figure 2).
The remaining tumours affected entire and nulliparous females. The mammary tumours were histologically classified according to the WHO histological classification of mammary tumours of the dog and cat (Misdorp et al, 1999). Three out of ten tumours were benign (two were simple adenomas and one was a benign mixed tumour). The seven malignant tumours identified during the study included six simple tubule-papillary carcinomas and one simple solid carcinoma (according with the WHO classification of mammary tumours in dogs and cats, simple carcinomas are divided into tubule-papillary, solid, and anaplastic types, reflecting increasing malignancy). Benign mammary tumours seemed to have a long disease-free period, whereas malignant mammary tumours showed a worse biological behaviour, mainly related with local recurrence and distant metastasis.
When the immunophenotype of neoplastic cells was studied, data revealed the glandular immunoprofile of all the tumours and suggested their ductal origin on the basis of consistent cytokeratin (CK) 20 expression. Interestingly, CK 7 was detected in basal/myoepithelial cells. The expression of CK 20 and CK 7 and the lack of expression of CK 8 in this species’ mammary gland tissue seem to represent a significant difference with respect to the CK profiles of other mammalian species. Further, all tumours were positive for type α oestrogen (ERα) and progesterone (PR) receptors (both PR and ERα expression was detected in the glandular epithelial cell compartment of all the guinea pig mammary gland tumours), while myoepithelial cells did not express hormone receptors, suggesting a role for steroid hormones in the development of these neoplasias in guinea pigs.