16 Oct 2017
The second of a two-part Exotic Encounters article by Elisabetta Mancinelli focuses on rats, looking at the latest findings in the species.
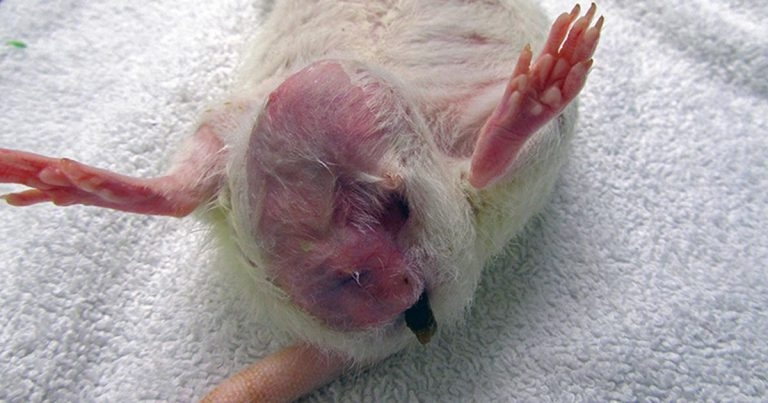
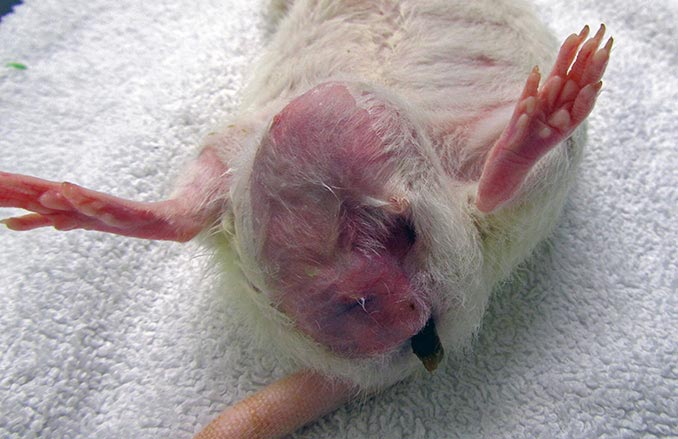
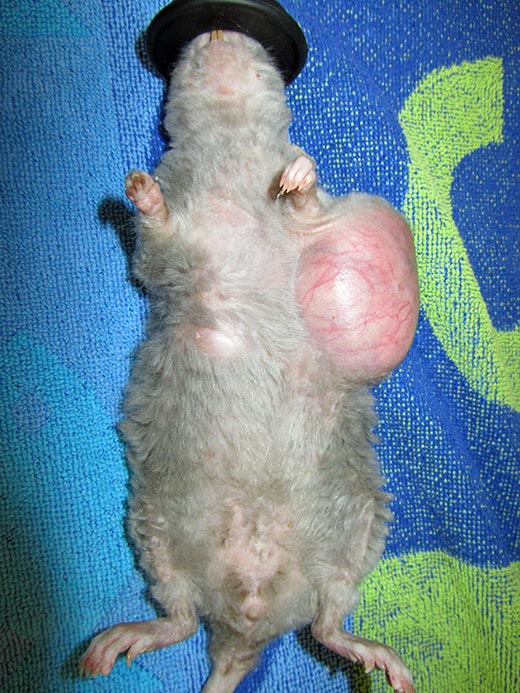
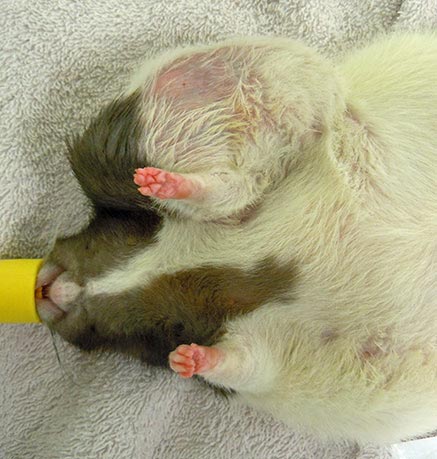
Rats have six pairs of mammary glands. The mammary tissue has an impressive extension from the neck to the inguinal region. These pictures show three different pet rats with mammary neoplasia developed along different body sections from the neck to the inguinal area.
Following on from the first part of this article, which looked at spontaneous mammary tumours in rabbits and guinea pigs, this second part will focus on rats, looking at the latest findings in this species (Vergneau-Grosset et al, 2016). Please refer to the literature provided for more detailed information.
Rats have six pairs of mammary glands (three thoracic, one abdominal and two inguinal pairs), with an impressive extension of mammary tissue from the neck to the inguinal region (Popesko et al, 2002; O’Malley, 2005).
Spontaneous mammary tumours are reported with an incidence of 30% to 67% in female Sprague Dawley rats (Dinse et al, 2010), with fibroadenomas being the most common spontaneous SC tumours (Toft, 1992) and adenocarcinomas representing less than 10% of cases (Hotchkiss, 1995).
In one study (Hotchkiss, 1995), although surgical excision of SC masses did result in improved welfare of affected laboratory rats, survival rates did not differ between rats that underwent surgical excision and those that didn’t. However, despite the reportedly high recurrence rate of mammary fibroadenomas, general consensus still considers surgical excision the best approach in animals that are suitable anaesthetic candidates. In the majority of these cases, extensive diagnostic testing is not performed prior to surgery and excised neoplasias are not histologically analysed on the assumption the mass is likely to be benign (Brown and Donnelly, 2011).
Mammary gland fibroadenomas are often found in association with other abnormalities, but specific data is lacking in pet rats. The association between mammary fibroadenomas and prolactinaemia (secondary to a reduced secretion of dopamine from the pituitary gland, low progesterone concentration, constant oestrus or cessation of ovulation in geriatric female rats) has been described in some laboratory strains (Nagasawa and Morii, 1981; Welsch et al, 1975; Tejwani et al, 1991; Mayer et al, 2011).
Prolactin-secreting pituitary tumours are often seen in laboratory rats with mammary tumours, but whether this may be an incidental finding considering the high incidence of both tumour types has not been fully debated (Hotchkiss, 1995). Similar tumours have been reported in pet rats presented for neurological signs, but the association with mammary tumour development was not remarked (Mayer et al, 2011; Vannevel, 2006).
It is reported ovariohysterectomy can help prevent the development of spontaneous mammary gland tumours. Their incidence rate was significantly less in surgically altered 90-day-old laboratory rats compared to older sexually intact females, whereas it was reduced from 73.8% to 5.3% if surgery was performed between five and seven months of age (Hotchkiss, 1995; Planas-Silva et al, 2008).
Ovariectomised Sprague Dawley rats also seem to have a low prevalence of pituitary tumours (Hotchkiss, 1995). The effect of ovariohysterectomy at the time of mammary tumour resection has not been thoroughly investigated in a controlled study. It is common opinion ovariectomy in laboratory rats leads to regression of chemically induced mammary tumours. However, Thordarson et al (2001) reported 90% of rats that underwent ovariectomy, in conjunction with surgical resection of a chemically induced mammary gland tumour, showed recurrence within two months and, in half of these cases, tumours were aggressive and lacked oestrogen receptors. If extensive, although controversial, data exists for laboratory rats, information regarding prevalence, histologic features, concurrent abnormalities and outcomes need to be collected and evaluated for pet rats.
These were the objective of a study performed by Vergneau-Grosset et al (2016), which retrospectively analysed medical records of 280 rats, presented for various reasons to a veterinary teaching hospital over a 25-year period. A total of 100 of these animals (17 males, 1 neutered male, 77 females and 5 spayed females) had at least one SC mass at time of presentation. Overall, results showed a prevalence of 63% for benign lesions and 25% for malignant ones. In 53% of cases, a mammary gland fibroadenoma was identified, followed by mammary gland carcinoma (12%), with 28% of all cases evaluated initially for reasons other than a SC mass. This puts emphasis on the importance of a complete physical examination in these species and suggests histological examination of the excised masses should always be performed.
Age was not associated with histologic diagnosis. Rats diagnosed with fibroadenomas were aged between 8 and 54 months, while age ranged between 12 and 36 months for rats with mammary carcinomas. Median age for diagnosis was 24 months for both groups. Fibroadenomas were more frequently seen in the axillary and ventral areas where mammary tissue is more extensive, although this may not be reflective of a real association between tumour type and location, considering the small number of tumours examined. Fibroadenomas also developed more frequently in sexually intact females and males compared to neutered animals. However, three spayed females developed mammary gland fibroadenomas. This can be explained by the fact ovariectomy reduces, but does not completely eliminate the risk of tumour development or the fact a subclinical mammary tumour may have already been present when the rats underwent ovariohysterectomy and may have continued to develop afterwards.
Similar to laboratory rats, the prevalence of pituitary neoplasia did not differ between pet rats with or without mammary fibroadenoma in the study. Therefore, it remains unclear whether pituitary tumours may have a role in the development of mammary gland fibroadenomas and reproductive tract disorders.
Several reproductive tract disorders were identified in 40% of the rats with mammary fibroadenomas that underwent a necropsy. More specifically, the four rats that had uterine cystic endometrial hyperplasia (the most common reproductive disorder seen) also had a mammary fibroadenoma and three out of four also had a pituitary tumour. This study could not evaluate whether a common link existed between these conditions. However, considering the high frequency with which female rats with mammary gland neoplasia also have concurrent reproductive tract disorders, ovariohysterectomy could be indicated and recommended.
The following treatments were implemented in the rats in the study; surgical excision, chemotherapy, surgical or chemical neutering with a GnRH agonist, and use of an antiprolactinaemic drug.
A total of 20 rats underwent successful excision of an initial mammary gland fibroadenoma – eight out of the 15 rats that underwent surgical excision of the mass without any other adjunct treatment developed a subsequent tumour within a median of 4.5 months. The median survival time of these 15 rats was 7.4 months after excision of the initial mammary mass. A total of 3 females that also underwent an ovariohysterectomy, alongside surgical excision of the SC mass, developed subsequent tumours, 6 out of 13 rats with mammary gland carcinoma underwent surgical excision and four of them developed subsequent tumours. The median survival time for these 6 rats was 4 months after initial examination and 4 of them developed metastasis to regional lymph nodes; 2 rats with mammary fibroadenomas also received cabergoline and deslorelin.
Prolactin antagonists are reported to prevent the development of spontaneous mammary fibroadenomas and decrease the size of some experimentally induced mammary neoplasia in some strains of laboratory rats, despite the fact hormonal receptors present on mammary gland tumours of pet rats have not been clearly identified. However, tumours recurred within one to nine weeks after discontinuation of treatment. The use of deslorelin as an adjunct treatment for mammary gland fibroadenoma has not been described. Implantation of a 4.7mg deslorelin implant leads to suppression of serum 17β oestradiol and progesterone for up to 12 months, and oestrogen and prolactin are considered important carcinogenetic promoting factors for the development of some types of tumours.
In the study, in an entire female rat with mammary fibroadenomas, which received two 4.7mg deslorelin implants, 15 months apart, and cabergoline for 2 weeks (Grosset et al, 2012; refer to the main articles cited for details of the protocol used), the mass appeared to remain stable after the second implant for 5 months, after which time the mass began to increase in size. It is unknown whether this may have been due to loss of efficacy by the implant, decreased response by the tumour after repeated implantation or evolution of the tumour receptors.
Another entire female rat received only one implant five weeks following surgical excision of a mammary fibroadenoma. It did not develop further masses in the subsequent 12 months, at which point it was euthanised for unrelated reasons.
Intralesional cisplatin was used in one case only; therefore, the authors could not make any suggestions regarding this treatment option.
It is difficult to draw any conclusion about the use and efficacy of hormonal therapy or the usefulness of surgical excision based on the relatively low numbers presented in this study. Certainly, this study presented several limitations and additional information is required before recommendations are able to be made, but these preliminary findings may be useful when discussing treatment options and relative outcomes.