17 Jul 2017
Studies into pet rodent species
Elisabetta Mancinelli reports on species that have been increasingly studied in the past decade.
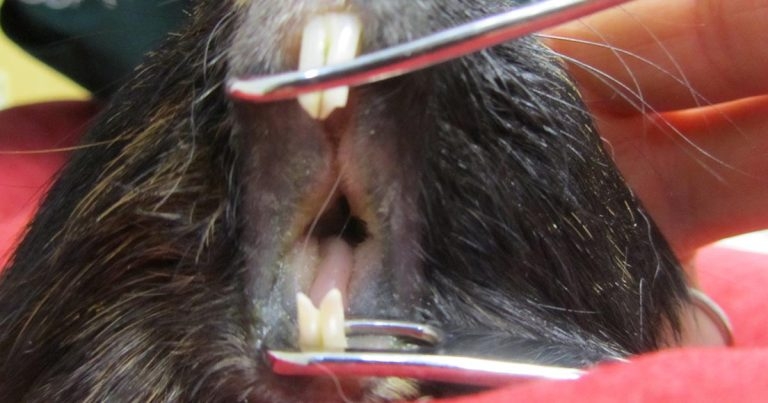
Figure 1. A guinea pig with advanced dental disease, anaesthetised for oral examination. Dental disease was the most commonly diagnosed in pet guinea pigs.
Many rodent species, often used as animal models in research, are commonly kept as pets.
The increasing awareness of veterinary professionals towards these species has resulted in an increase in research in the past decade, which is helping, more often, achieve a definitive diagnosis and establish a more targeted treatment, ultimately improving standards of care. As practising vets, we should look with interest into these studies, as results may be of clinical interest and applicable in our everyday job.
Diseases in pet guinea pigs
Several publications exist regarding the prevalence of specific diseases or organ pathology in guinea pigs (Gipson and Wagner, 1986; Böhmer and Köstlin, 1988; Fehr and Rappold, 1997; Jekl et al, 2008; Williams and Sullivan, 2010).
In a retrospective study (Minarikova et al, 2015), disease prevalence in 1,000 guinea pigs (544 males and 456 females) kept as pets was described. Animals were divided into three groups based on their age:
- Young (less than two years old). Total number: 561 (296 males and 265 females).
- Middle age (between two and five years old). Total number: 376 (216 males and 160 females).
- Old (more than five years old). Total number: 63 (34 males and 29 females).
Dental disease was the most commonly diagnosed disease (36.3%), with higher prevalence in the middle age group (two to five years old) and in males (P<0.001) rather than females (Figure 1).
Changes most commonly seen included apical and coronal tooth elongation, uneven cheek teeth or incisor occlusal surface, tooth fractures, structural tooth changes or periapical pathology. Periodontitis and presence of hair in the gingival sulci were seen in 10% of guinea pigs with dental disease. Odontogenic abscesses were diagnosed in 3.1% (31 out of 1,000) of cases. Iatrogenic incisor malocclusion, typically caused by veterinary general practitioners using unsuitable tools for incisor clinical crown height correction, was seen in 2.7% (27 out of 1,000) of animals, and was generally characterised by evidence of tooth fracture, uneven occlusal surface, pathological tooth movement, pulpal opening and bleeding.
Müller et al (2015) suggested factors other than diet abrasiveness, such as mineral imbalances and, in particular, hereditary malocclusion, are more likely causes for dental problems observed in this species.
Skin disease was the second most common disorder, with a prevalence of 33.3%, which was higher in male guinea pigs and in animals younger than two years old. Ectoparasites were more commonly seen (Trixacarus caviae, Gliricola porcelli, Chirodiscoides caviae, Gyropus ovalis, Ctenocephalides felis and Ornithonyssus bacoti).
Dermatophytosis was more often described in middle-aged guinea pigs, while skin tumours were more frequently diagnosed in animals younger than two years old. Young guinea pigs have an underdeveloped immune system and a lower concentration of fungistatic fatty acids in their sebum (Vangeel et al, 2000), which could explain the higher prevalence of these disorders.
Reproductive disorders were seen in 15.8% of cases. Ovarian cystic disease was most commonly seen (21.9%), with higher prevalence in females more than two years old (Figure 2). The incidence of tumours affecting the reproductive tract was significantly higher in females than males. Mammary gland tumour prevalence was significantly higher in the older age group and in males.
Other common health disorders included ophthalmological disorders (15%) and gastrointestinal disturbances (13.1%). In particular, “simple” gastric dilatation, and gastric dilatation and volvulus (GDV), were seen in 10 and 34 cases respectively. The prevalence of GDV was significantly higher in guinea pigs more than two years old. Factors predisposing to this condition are still unknown and no direct association with either dietary change or other possible causes of this life-threatening condition was seen during this study.
Respiratory disease was only recorded in 4% of cases in the study, which may be due to the reasonably good husbandry conditions animals were kept in, which reduces risks of pneumonia.
Disorders of the urinary system were diagnosed in 42 guinea pigs; more commonly in females and animals more than two years old. Uroliths mainly contained calcium salts.
Femorotibial OA was the most common musculoskeletal disorder, more often diagnosed in older animals. A lack of, or excess, dietary ascorbic acid may result in osteoarthrosis, suggesting appropriate vitamin C supplementation should be done in this species. Otitis media was diagnosed based on thickening of the tympanic bulla wall in 62 cases. Otitis media in guinea pigs mostly follows a subclinical course, but the clinical impact of this disease is, to date, unknown (Martorell and Vilalta, 2013). Only 8.1% of guinea pigs were healthy; therefore, it is always important to perform a thorough clinical examination and educate clients about the prevention of the most common diseases in guinea pigs.
Odontogenic abscesses
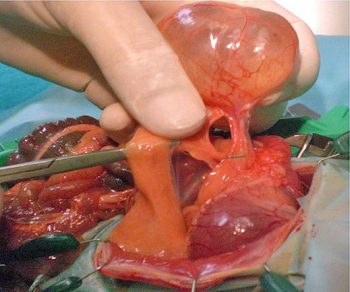
The aforementioned study (Minarikova et al, 2015) confirmed dental disease was the most common condition seen in 36.3% of guinea pig cases.
Odontogenic abscesses, always diagnosed along with dental disease, were seen in 31 out of 1,000 cases and represented a serious health problem to guinea pigs. Clinical manifestation of this condition may include facial masses, respiratory disease and/or exophthalmos (retrobulbar mass) depending on the affected jaw (Minarikova et al, 2015). Treatment may involve a combination of the following:
- extraction of the affected tooth/teeth
- removal of the affected bone
- thorough wound debridement
- supportive care
- analgesia and antibiotic administration
The use of antibiotics should be based on bacterial culture and sensitivity testing (Capello, 2008). However, information about microbial flora of odontogenic abscesses in guinea pigs is limited, making interpretation of bacteriology results challenging.
A prospective study was therefore performed to describe the microbial flora of odontogenic abscesses associated with osteomyelitis in 73 pet guinea pigs (35 females and 38 males, aged from three months old to seven years old) with relative antibiotic sensitivity testing, to be able to make recommendations for practitioners on the antibiotics of first choice (Minarikova et al, 2016). Samples (pus, capsule and affected tooth/bone) for bacteriological examination were collected during the surgical procedure performed to treat the abscess. The most common isolates were Bacteroides fragilis in 12.8% (6 out of 47) of cases, Pasteurella multocida in 10.6% (5 out of 47) and Peptostreptococcus anaerobius in 8.5% (4 out of 47). Aerobic bacterial species were only isolated in 29.2% (7 out of 24) of cases, anaerobic bacteria was only isolated in 33.3% (8 out of 24), and mixed infection with anaerobic and aerobic bacterial species was seen in 37.5% (9 out of 24).
Similarly, in rabbits, the most common cause of odontogenic abscesses was a mixed infection, particularly with anaerobic Gram-negative rods (predominantly Fusobacterium nucleatum), anaerobic Gram-positive, non-sporulating rods (predominantly Actinomyces species) and aerobic Gram-positive cocci (especially the genus Streptococcus milleri; Tyrrell et al, 2002). In guinea pigs, aerobes (n = 20) were sensitive to enrofloxacin and marbofloxacin in 100% of samples, benzylpenicillin potassium (penicillin G; PNCG) in 90%, cefalotin in 85%, amoxicillin/clavulanate in 75%, doxycycline in 70%, gentamicin in 65% and trimethoprim-sulfamethoxazole in 55%. Anaerobes (n = 27) were sensitive to amoxicillin-clavulanate in 100% of cases, clindamycin in 96.3%, metronidazole in 92.6%, PNCG in 92.6% and cefalotin in 74.1%.
As guinea pigs are strictly herbivorous animals in which penicillins and clindamycin can potentially cause clostridial infections and toxaemia, based on the results of this study the recommended antibiotic treatment for odontogenic abscesses is a combination of fluoroquinolones (enrofloxacin and marbofloxacin) with nitroimidazole antibiotics (metronidazole), while waiting for sensitivity results.
Authors have also pointed out Pseudomonas aeruginosa is a potential nosocomial pathogen (Lister et al, 2009). As some of the other bacteria (Bacteroides species, Prevotella oris and Fusobacterium species) isolated in the study may have zoonotic potential and cause respiratory, skin, brain or mouth cavity infections, it is necessary to use personal protective equipment during surgery. Thorough disinfection of the hospital environment where the sick guinea pigs are kept (cage/box) is also advised and clients should be informed about any possible zoonotic potential of isolated bacteria from odontogenic abscesses in this species.
Urinalysis in chinchillas
Urinalysis (including determination of urine specific gravity; USG), chemical analytes (by use of a dipstick and sediment microscopic examination) can be helpful in diagnosing both urinary tract and systemic disorders. Penile fur rings, urinary tract or urogenital diseases are uncommon in chinchillas (Mans and Donnelly, 2012).
In a retrospective review of necropsy results for 202 chinchillas, only 5 had renal disease (Lucena et al, 2012). Chinchillas can also develop urinary calculi, which are more commonly seen in males, with haematuria being the most frequently observed clinical sign (Mans and Donnelly, 2012). Within the order caviomorpha, urinalysis results for pet rodents have only been reported in guinea pigs (Bishop et al, 2010; Rueloekke et al, 2016). A study was therefore undertaken, including 41 clinically normal chinchillas (29 females and 12 males) exposed during a breeder exposition, to collect urinalysis data on voided urine samples including USG (measured before and after centrifugation with a handheld veterinary refractometer), urine dipstick analysis, microscopic sediment examination, urine sulfosalicylic acid (SSA) precipitation test and quantitative protein analysis (Doss et al, 2016). The urine varied greatly in colour.
Pigments in the diet have been associated with urine colour in chinchillas, as in rabbits (Banks et al, 2010; Melillo, 2007). Mostly, the samples were clear. The turbid samples had a large number of crystals. Unlike guinea pigs and rabbits, which excrete large amount of calcium in their urine, chinchillas excrete calcium primarily through faeces and do not rely on urinary secretion of calcium to maintain calcium homeostasis (Hagen et al, 2014). In the study, urine samples that were dark in colour generally also had a higher USG than lighter-coloured samples. The USG measured had a wide range (1.010 to more than 1.060), although a large proportion (17 out of 41; 41%) of the chinchillas had a USG less than 1.050. The USG before centrifugation did not differ significantly from that after centrifugation, even in those samples with visible sediment; therefore, centrifugation of chinchilla urine samples prior to measurement of USG with a refractometer is not considered necessary.
Protein was detected in all urine samples on dipstick analysis. Several exotic mammals including hamsters, mice, rats, gerbils and rabbits are reported to have small amounts of protein in their urine (Fisher, 2006). However, unexpectedly, 98% of the animals had more than trace amounts of dipstick protein contents, which may be a function of the fairly high USGs for this population. This prompted further tests, including the SSA precipitation test (a turbidimetric screening test for proteinuria, commonly used to verify positive dipstick protein results) and quantification of urine protein concentration on the samples for which sufficient volume was available (37 and 18 samples, respectively). The results suggested the dipstick protein and the SSA test results were not accurate. The urine dipsticks used in the study are marketed for use in human urine samples, and values of 1+, 2+, 3+ and 4+ are approximately equivalent to urine protein concentrations of 30mg/dL, 100mg/dL, 300mg/dL and greater than or equal to 2,000mg/dL, respectively. For the 18 urine samples that underwent quantitative protein analysis, the maximum urine protein concentration was 87mg/dL, which suggested the maximum dipstick protein result should have been 1+. In the study, USG was positively correlated with the dipstick protein results, which might have contributed to the unreliability of the dipstick protein results.
In small animals, a high USG and alkaline urine are associated with false-positive urine dipstick protein results (Grauer, 2007). The recorded pH for all samples was 8.5, which was the upper limit of detection for the reagent strip. False-negative SSA test results can occur because grading of turbidity is subjective and variation between individual readers is likely (Grauer, 2007). This was considered unlikely to have occurred in this study.
High alkaline urine may also lead to false-negatives, which may have affected the SSA test results in this study. In Sprague-Dawley rats, it is reported the SSA precipitation test should not be used to verify urine dipstick protein results (Reagan et al, 2007). Glucose or ketones, but not both, were detected in 5 and 6 samples, respectively. In chinchillas, ketoacidosis can develop as a sequela to anorexia. It is possible stress associated with transport and exhibition might have resulted in reduced food intake with subsequent ketones production and urinary excretion. Stress could have also caused hyperglycaemia, which subsequently led to glucosuria, although an association between stress and hyperglycaemia has not been clearly established for chinchillas.
Ketonuria, in conjunction with glucosuria, has been reported in chinchillas with hepatic lipidosis (Mans and Donnelly, 2012). In this study, subclinical disease could not be ruled out, which represents a limitation. Crystals were observed in 28 of 41 (68%) samples; 27 of those samples contained amorphous crystals, but, to date, their significance is unknown in this species.
Rare epithelial cells, red blood cells and white blood cells were also seen in many samples, and likely represented non-pathological findings.
References
- Banks RE, Sharp JM, Doss SD et al (2010). Exotic Small Mammal Care and Husbandry. Wiley-Blackwell, Hoboken: 125-136.
- Bishop CR, Fischer J, Brossoit A et al (2010). Standardization of renal physiology parameters in guinea pigs via urinalysis, Proceedings of the 31st Annual Association of Avian Veterinarians (AAV) Conference and Expo, AAV, San Diego: 49-52.
- Böhmer E and Köstlin R (1988). Zahnerkrankungen bzw. -anomalien bei Hasenartigen und Nagern, Der praktische Tierarzt 69: 37-50.
- Capello V (2008). Clinical technique: Treatment of periapical infections in pet rabbits and rodents, J Exot Pet Med 17(2): 124-131.
- Doss GA, Mans C, Houseright RA et al (2016). Urinalysis in chinchillas (Chinchilla lanigera), J Am Vet Med Assoc 248(8): 901-907.
- Fehr M and Rappold S (1997). Urolithiasis in 20 guinea pigs (Cavia porcellus), Tieraerzliche Praxis 25: 543-547.
- Fisher PG (2006). Exotic mammal renal disease: diagnosis and treatment, Vet Clin North Am Exot Anim Pract 9(1): 69-96.
- Gipson SW and Wagner JE (1986). Cryptosporidiosis in guinea pigs: a retrospective study, J Am Vet Med Assoc 189(9): 1,033-1,034.
- Grauer GF (2007). Measurement, interpretation, and implications of proteinuria and albuminuria, Vet Clin North Am Small Anim Pract 37(2): 283-295.
- Hagen K, Clauss M and Hatt JM (2014). Drinking preferences in chinchillas (Chinchilla laniger), degus (Octodon degu) and guinea pigs (Cavia porcellus), J Anim Physiol Anim Nutr (Berl) 98(5): 942-947.
- Jekl V, Hauptman K and Knotek Z (2008). Quantitative and qualitative assessments of intraoral lesions in 180 small herbivorous mammals, Vet Rec 162(14): 442-449.
- Lucena RB, Giaretta PR, Tessele B et al (2012). Diseases of chinchilla (Chinchilla lanigera), Pesq Vet Bras 32(6): 529-535.
- Lister PD, Wolter DJ and Hanson ND (2009). Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms, Clin Microbiol Rev 22(4): 582-610.
- Mans C and Donnelly TM (2012). Disease problems of chinchillas. In Quesenberry K and Carpenter J (eds), Ferrets, Rabbits, and Rodents. Clinical Medicine and Surgery (3rd edn), Elsevier, St Louis: 311-332.
- Martorell J and Vilalta L (2013). Subclinical middle ear disease in guinea pigs, Proceedings of the 1st International Conference on Avian, Herpetological and Exotic Mammal Medicine, Wiesbaden, Germany: 115.
- Melillo A (2007). Rabbit clinical pathology, J Exot Pet Med 16(3): 135-145.
- Minarikova A, Hauptman K, Jeklova E et al (2015). Diseases in pet guinea pigs: a retrospective study in 1,000 animals, Vet Rec 177: 200.
- Minarikova A, Hauptman K, Knotek Z et al (2016). Microbial flora of odontogenic abscesses in pet guinea pigs, Vet Rec 179(13): 331.
- Müller J, Clauss M, Codron D et al (2015). Tooth length and incisal wear and growth in guinea pigs (Cavia porcellus) fed diets of different abrasiveness, J Anim Physiol Anim Nutr (Berl) 99(3): 591-604.
- Reagan WJ, VanderLind B, Shearer A et al (2007). Influence of urine pH on accurate urinary protein determination in Sprague-Dawley rats, Vet Clin Pathol 36(1): 73-78.
- Rueloekke ML, Spangsberg R and Koch J (2016). Physiologic serial changes in the visual appearance of urine samples from individual pet guinea pigs (Cavia porcellus), BSAVA Congress Proceedings, BSAVA, Birmingham: 553-554.
- Tyrrell KL, Citron DM, Jenkins JR et al (2002). Periodontal bacteria in rabbit mandibular and maxillary abscesses, Journal Clin Microbiol 40(3): 1,044-1,047.
- Vangeel L, Pasmans F, Vanrobaeys M et al (2000). Prevalence of dermatophytes in asymptomatic guinea pigs and rabbits, Vet Rec 146(15): 440-441.
- Williams D and Sullivan A (2010). Ocular disease in the guinea pig (Cavia porcellus): a survey of 1,000 animals, Vet Ophthalmol 13: 54-62.
