25 Oct 2017
The rodent patient: how to deal with common emergencies
Rodent medicine has developed greatly in recent years and, as Livia Benato explains, many treatments and diagnostic tests can now be offered for these interesting animals.

Figure 1. Oxygen therapy of a rat – the chest is slightly raised to improve oxygenation.
Emergency and critical care of rodents is an exciting challenge as prey species, such as these, may disguise obvious clinical signs at presentation.
However, a good knowledge of the signs of pain and distress in these species, coupled with a familiarity of common presentations, can facilitate the treatment of these pets.
Taking the call
In an emergency, assessment and management of the patient begins during the first telephone call to the practice. Here, it is important to not only have an idea of the severity of the clinical signs, but also be able to support the owner – advising him or her to bring the animal to the hospital immediately, including how to transport it, if necessary.
A rodent not eating or passing faeces is as much of an emergency as one that is collapsed or struggling to breathe.
Be prepared
In the meantime, the necessary equipment to deal with the emergency can be prepared ready for the little patient’s arrival.
As for other companion animals, such as cats and dogs, it is advisable to have a “crash box” stocked with the necessary equipment, ready to use in case of emergency. This should contain equipment for cardiovascular resuscitation and immediate critical care, such as first aid medications, IV catheters, fluids and airway devices.
Most importantly, it should include a drug chart with dosages depending on the species and weight. These are small animals and, often, dilution of the drugs may be necessary – therefore, a chart will ease preparation and administration.
At the clinic
The rodent should first be stabilised. If the animal is having a seizure or has collapsed, immediate intervention is needed.
In cases of respiratory distress, oxygenation in a dark, quiet room should be given before any handling to minimise stress in an already unstable patient.
Once the rodent has been stabilised, the history should be taken – including management, nutrition and previous problems – so the severity of the condition and the presence of concurrent problems can be established.
A physical examination should then be performed, with the animal also observed for subtle signs of discomfort or pain. Further investigations can then be advised and performed.
Seizures
Seizures are not common in rodents, but when they occur – as in cats and dogs – extracranial and intracranial causes need ruling out.
Problems that can lead to seizures include:
- middle ear infection
- trauma
- hypoglycaemia
- insulinoma
- pituitary tumours (common in older rats)
- toxicity due to lead and pesticides
Treatment generally consists of controlling the seizure using diazepam intravenously (Oglesbee, 2011), while investigating the primary cause.
In guinea pigs, seizures can also be caused by intense pruritus due to severe mite infestation. The animal is generally presented due to seizure, but alopecia, crusting and scratching are also noticed at physical examination. It’s likely handling has led to the pruritus, therefore triggering the seizures.
Skin scraping should be performed to confirm the diagnosis, but a presumptive diagnosis can already be made based on the history and physical examination.
Mite infestation is often secondary to stress and chronic disease, and treatment can be started by administration of selamectin spot-on. Further treatment is often necessary with antibiotics and analgesia to treat secondary bacterial infection and discomfort.
Collapsed or unconscious
If the animal is presented collapsed or unconscious on arrival, CPR may be necessary to prevent pulmonary and cardiac arrest – and it is important to act quickly.
If the rodent has stopped breathing, the oral cavity should be checked for any foreign body – such as pieces of food – that may block the airways.
An otoscope can facilitate this procedure, as a rodent’s mouth is long and narrow. The airways should then be secured and ventilation started.
In the case of guinea pigs, it is possible to intubate using a small endotracheal tube. For smaller rodents, such as rats and hamsters, an emergency airway can be obtained using a close-fitted face mask.
Once the airways are secured, the rodent can be manually ventilated using 100% oxygen attached to a breathing system, such as a T-piece, or using room oxygen (21%) via a manual resuscitator. Doxapram can then be administered.
In cases of cardiac arrest, cardiac massage can be started, placing the thumb and the fingers around the chest and starting regular compressions. The rodent chest is very small compared to a cat or dog, and the fingers should be placed cranially. Epinephrine can be administered either intravenously or via the intraosseous route at the same time.
Cardiac activity can be monitored using a stethoscope and a Doppler probe. In cases of bradycardia, either atropine or glycopyrrolate can be administered.
Breathing issues
Breathing difficulty, or dyspnoea, is a common presentation in rodents – especially in rats – and it is generally associated with serious conditions. The most common causes of dyspnoea in rodents are upper and lower respiratory infections, as well as cardiac diseases, such as dilated cardiomyopathy.
Rodents presenting with open-mouth breathing have a guarded to poor prognosis. This is because rodents are obligate nasal breathers, so to breathe through the mouth they have to make an effort to dislodge the long palate. This is shown only during severe respiratory impairment.
Oxygenation can be provided via a small oxygen tent or face mask (Figure 1). The tent provides the most hands-off approach and the rodent can be monitored without restraint or handling, while an induction chamber or face mask can be useful if the oxygen tent is not available or ready.

However, close proximity to people can further stress the animal, so a towel can be used to cover part of the mask or chamber.
When possible, the oxygen should be moistened using a nebuliser attached to the oxygen supply – this will minimise moisture loss of the airways that would lead to dryness of the mucous membranes and irritation.
In cases of nasal discharge, it will also keep the discharge moist, allowing the animal to sneeze and breathe better.
If the oxygen provided cannot be moistened, it would be ideal to nebulise the animal with saline solution. However, the noise of the nebuliser can be stressful, so the benefit of nebulisation should be assessed case by case.
A warm environment should be maintained at all times, while further treatment and care of the patient depends on the cause of the dyspnoea.
Physical injuries
Physical injuries – such as wounds, sores, bruises and limb fractures – are generally caused by predators and cages with meshed floors. However, they can also be caused by improper handling, crushing and dropping of the animal. Bite wounds, meanwhile, can be caused not only by predators – such as cats and dogs – but also by other rodents.
Rodents can be very territorial, especially if of the same sex or recently introduced. The resulting aggression can lead to severe bruising and wounds, meaning the patient is likely to be presented with severe bleeding and hypovolaemic shock.
At presentation, the main clinical signs of shock are bradycardia, bradypnoea and hypothermia. However, tachycardia and tachypnoea can also be apparent. The mucous membranes are pale, the capillary refill time is decreased or absent and the pulse is weak due to low blood pressure. In severe cases, the rodent is lethargic, or arrives collapsed.
Treatment involves raising blood pressure and volume, and limiting the tissue damage that occurs secondary to reduced tissue perfusion.
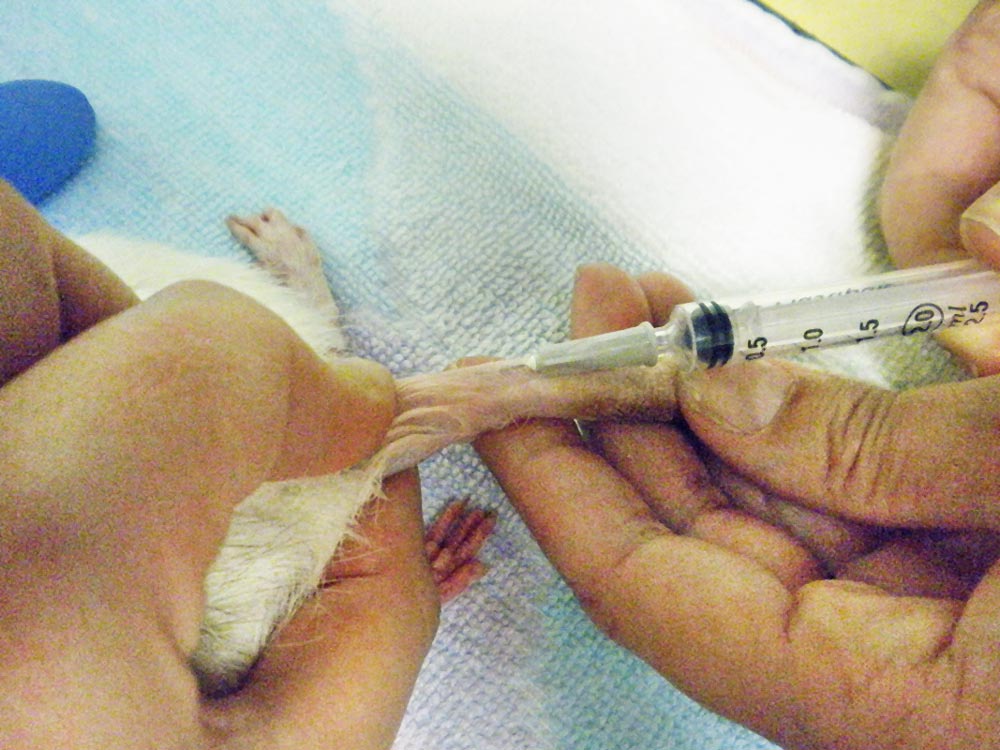
Aggressive fluid therapy is administered intravenously (Table 1), or via the intraosseous route in small or collapsed animals, using either isotonic crystalloids at a rate of up to 60ml/kg over one hour, or a combination of crystalloids (boluses of 10ml/kg to 15ml/kg) and colloids, such as hydroxyethyl (boluses of 3ml/kg to 5ml/kg over 10 min), until hypotension is treated and blood pressure is within normal limits (Lennox, 2008).
| Table 1. Veins recommended for IV catheter placement and blood sampling | |
|---|---|
| Species | Veins |
| Guinea pigs and chinchillas | • Lateral saphenous • Cephalic vein • Jugular vein • Cranial vena cava (guinea pigs under sedation) |
| Rats and gerbils | • Lateral saphenous • Lateral tail vein (Figure 2) |
| Hamsters and degus | • Lateral saphenous |
|
|
|
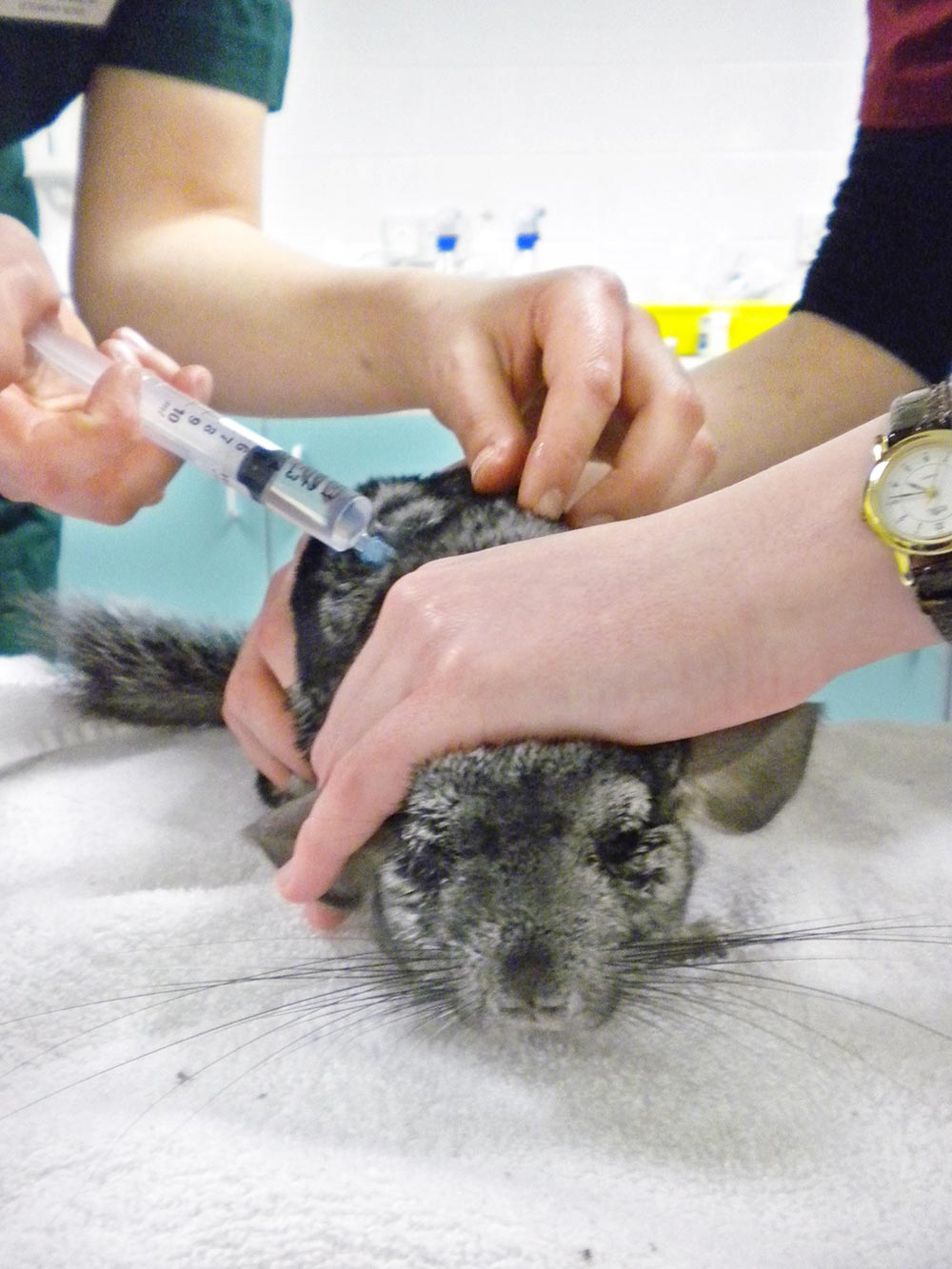
At the same time, it is also important to gradually warm up the rodent and address the cause of hypovolaemic shock to prevent further worsening of the animal. Maintenance fluids should be continued at 100ml/kg/day (Figure 3).
Pain and analgesia
Analgesia is an important aspect of the critical management of these animals. NSAIDs and opioids are the most common and safest drugs available (Wenger, 2012; Table 2).
Among the NSAIDs, meloxicam is available in injectable and oral formulations, allowing the continuation of therapy after the initial treatment.
The opioids buprenorphine and butorphanol can be used intramuscularly or subcutaneously. Buprenorphine can also be administered sublingually.
| Table 2. Analgesia in rodents (extrapolated from BSAVA Small Animal Formulary and Veterinary Information Network Formulary for Exotic Animals) | |
|---|---|
| Drug | Dosage |
| Meloxicam | • Guinea pigs: 0.2mg/kg to 0.5mg/kg SC, PO, SID • Chinchillas: 0.3mg/kg to 0.5mg/kg SC, PO, SID, BID • Rats, hamsters, mice and gerbils: 1mg/kg to 2mg/kg SC, PO, SID |
| Carprofen | 2-5mg/kg IV, SC, PO, SID (or half a dose BID) |
| Buprenorphine | 0.01mg/kg to 0.05mg/kg IM, SC, q6-12h |
| Butorphanol | • Chinchillas and guinea pigs: 2mg/kg q4h • Gerbils, hamsters and rats: 1mg/kg to 5mg/kg SC, q4h |
| Tramadol | • Guinea pigs: 4mg/kg to 5mg/kg PO, BID • Rats: 10mg/kg to 20mg/kg SC, PO, TID, BID |
| Gabapentin | • Hamsters: 50mg/kg PO, SID • Rats: 30mg/kg PO, TID |
Rodents are prey species and tend to hide signs of pain, making its presence more difficult to assess. However, a good knowledge of normal rodent behaviour will alert clinicians to the subtle changes resulting from pain. Owners should also be able to report changes in the animal’s routine and the presence of behaviours not present before the pain.
If in doubt, conditions considered painful for other companion animals, such as cats and dogs, should also be considered painful in rodents and treated appropriately.
Common signs of pain in rodents are:
- aggression and biting
- a hunched back
- reluctance to move
- teeth grinding
- closed eyes
- abnormal locomotion
- reduced appetite
- reduced faecal output
During hospitalisation, a record of abnormal behaviours should be kept. This can give indications if the analgesic protocol is having beneficial effects.
Conclusion
This article introduced the most common emergency procedures necessary to stabilise rodent patients at arrival. Once they are stable, further investigations are generally necessary to make a diagnosis and create a treatment plan.
It is also important to remember, while hospitalised, fluid therapy should be continued and assisted feeding started. A variety of critical care formulas for both omnivorous and herbivorous species should be available at the clinic.
- Some drugs mentioned in this article are used under the cascade.
References
- Bradley Bays T (2013). Recognising signs of pain and pain management in exotics, Proceedings of the 65th Canadian Veterinary Medical Association Convention.
- Hawkins MG and Graham JE (2007). Emergency and critical care of rodents, Veterinary Clinics of North America: Exotic Animal Practice 10(2): 501-531.
- Howell CM and Brown C (2008). Assisted feeding for small herbivores, Laboratory Animals 37(6): 251-252.
- Johnson DH (2013). Critical care in rodents and how to get them to survive, Proceedings of the American Board of Veterinary Practitioners Symposium, Phoenix.
- Lichtenberger M and Hawkins MG (2009). Rodents: physical examination and emergency care. In Keeble E and Meredith A (eds), BSAVA Manual of Rodents and Ferrets, BSAVA, Gloucester: 18-31.
- Lennox AM (2008). Critical rodents and other small exotic mammal emergencies, Proceedings of the North American Veterinary Community Conference, Orlando: 1,827-1,828. http://bit.ly/2kO1Ypq
- Meredith A (2015). BSAVA Small Animal Formulary Part B: Exotic Pets (9th edn), BSAVA, Gloucester.
- Oglesbee BL (2011). Exotic mammal emergencies, Blackwell’s Five-Minute Veterinary Consult: Small Mammal (2nd edn), Wiley Blackwell, Iowa.
- Wenger S (2012). Anaesthesia and analgesia in rabbits and rodents, Journal of Exotic Pet Medicine 21(1): 7-16.