17 Feb 2021
Bovine respiratory disease: causes, prevention and management
Nina Gammage discusses a challenging and ubiquitous disease in full before offering a case study for analysis.

Image: kelly marken / Adobe Stock

The inescapable interaction between pathogens, environment and calf factors often results in the development of the challenging and ubiquitous bovine respiratory disease complex (BRDC).
Primary lung damage – often initiated by respiratory viruses – promotes opportunistic bacterial infection, resulting in clinical pneumonia. Poor lung health – often predicated by inadequate housing – predisposes to disease and delays recovery.
This detrimentally affects welfare and has significant financial implications via immediate costs associated with the outbreak, ongoing reductions in growth rates and subsequent disease episodes. Respiratory disease costs the UK cattle industry an estimated £80 million annually – from £30 in mild cases, up to £500 if an animal dies (Scott, 2009). These ongoing costs can have a knock-on effect on farmers’ ability to implement strategies to prevent future outbreaks.
By considering the infectious agents, non-infectious components and control measures, a plan can be made to reduce the risk of BRDC and minimise the impact should outbreaks occur.
Primary pathogens
Infectious bovine rhinotracheitis (IBR) is commonly implicated in both BRDC and as a primary pathogen. It is caused by bovine herpes virus 1 (BHV-1), which persists in the population through a latent phase that is thought to occur in the trigeminal nerve (Andrews, 2004), resulting in clinically healthy carriers that may test serologically negative, but will intermittently shed virus in periods of stress.
The true prevalence of BHV-1 in the UK is unknown; however, in 1998 a study showed that 69% of 341 dairy herds had BHV-1 antibodies present in bulk milk ELISA (Paton et al, 1998). In outbreaks, morbidity can reach up to 100%, and mortality is quoted between 1% and 10% (Nandi et al, 2009). Experimentally, an incubation period of between 3 to 7 days has been proposed, although in outbreak situations 10 to 20 days is more common (Andrews, 2004).
Uncomplicated infection with bovine parainfluenza-3 (PI3), in contrast, does not usually produce severe disease. PI3 is an RNA virus and exclusively a respiratory pathogen, which rarely, if ever, causes systemic infection. Adult cattle in particular experience asymptomatic to mild infection (Campbell, 2015), and the important role of PI3 is to predispose immature cattle to infection by secondary viral and bacterial pathogens via two primary pathways. The first is through direct damage to respiratory clearance mechanisms and lung parenchyma, which promotes establishment of infection in the compromised parenchyma through transfer of bacteria from the upper respiratory tract. The second is that the immune system’s response to bacterial infection is reduced by viral infection (Czuprynski et al, 2004).
Bovine respiratory syncytial virus (BRSV) is an RNA virus that causes primary disease through the formation of syncytial cells via its cytopathic effect. Due to its predilection for the lungs, where it predisposes to secondary bacterial infection, BRSV is also a main component of BRDC. Maternally derived antibodies reduce the severity of pneumonia, but do not provide solid immunity. Outbreaks of BRSV are characterised by high morbidity, and mortality of up to 20% (Campbell, 2015).
The main routes of transmission of these primary pathogens are by aerosol and fomites contaminated with nasal secretions (MacLachlan and Duboui, 2017), and these represent critical areas for the control of BRDC. Early implementation of strict biosecurity measures can contain an outbreak to a shed and profoundly reduce morbidity. It is crucial to ensure all farm staff are involved and onboard, as biosecurity measures are only effective if carried out by everyone who enters or leaves the shed.
Husbandry
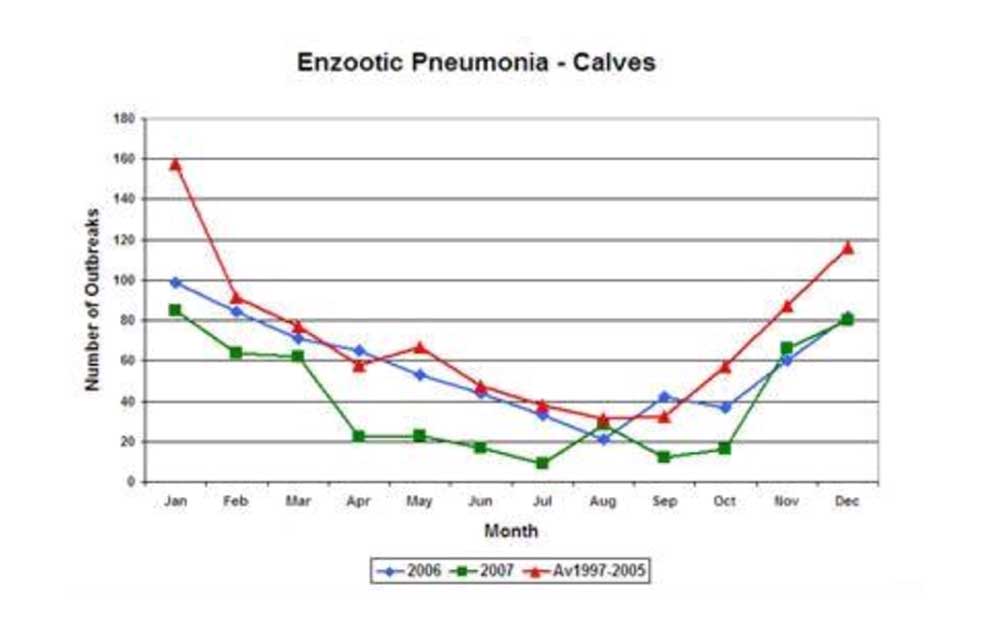
While it is universally accepted BRDC is an infectious complex, trials have failed to replicate the general clinical picture through exposure to individual viruses or bacteria (Jericho and Langford, 1978). Commonly implicated bacterial species are also easily cultured from the respiratory tract of normal cattle (Allen et al, 1992), indicating the aetiology of BRDC relies on non-infectious predisposing elements. Figure 1 demonstrates the seasonal nature of BRDC, with the majority of infections occurring within a month of housing. The stress of housing and mixing cattle of different ages in a shared airspace is a recognised precursor to pneumonia.
Environmental factors, such as ventilation and stocking rates, play an important role in lung health, and their significance cannot be overstated. In an outbreak scenario, drastic changes to building design are often not immediately practicable, but the following year’s health plan should consider housing improvements. Understanding a farm’s business plan and aims for the following five years can guide discussions about developments; for example, a new shed or ventilation system may be planned. Bear in mind, though, that once considered these changes may not be possible.
The Agriculture and Horticulture Development Board’s “Pneumonia MOT” explains calculations for ventilation and stocking in straightforward terms, and is an excellent starting point for discussions regarding shed design and planning.
Practical steps to address the environment can be taken in the immediate event of an outbreak. These include:
- Assessing the shed. Where are the calves gathering? Are they comfortably spaced or huddling next to the door where fresh air flows? Condensation inside the roof and cobwebs over outlets are further indicators of poor ventilation.
- Assessing the air quality at calf height. Can you comfortably breathe without coughing when bending down to calf height? A strong smell of ammonia can be alleviated by better ventilation (see later) or increasing bedding frequency.
- Improving drainage – do the squelch test. Can you kneel (without waterproofs) on the bedding without getting your knees wet? If not, the amount of bedding should be increased.
- Improving ventilation. If Yorkshire boarding is present, remove every second board or open doors at either end of the shed to encourage air to flow through. Be careful not to create draughts, though.
- Reducing stocking density. Split the calves into clinical and suspected subclinical cases. Remove clinical cases to a separate area – if a separate airspace is not present, move them to avoid nose-to-nose contact.
Calf factors
Maternally derived antibodies play a critical role in immunity against BRDC. Concentration of serum total protein influences the occurrence, age of onset and severity of pneumonia; however, this effect reduces as the calf ages (Arthur et al, 1998). The average costs of failure of passive transfer (FPT) per calf are estimated to be £53 and £71 for dairy and beef, respectively (Raboisson et al, 2016).
In young calves, checking total protein is an effective way to check for FPT, which is considered to have occurred if serum IgG concentration is below 10mg/mL (5.2g/dL; Faber et al, 2005).
In Scotland, farms should know their bovine viral diarrhoea (BVD) status as part of the BVD eradication scheme. BVD Free England is a voluntary scheme. The presence of persistently infected animals can cause transient infection in non-PI calves, resulting in immunosuppression and contributing to development of BRDC. A pneumonia investigation should include determining the BVD status of the affected animals with a view to opening a wider discussion, perhaps when the immediate situation is under control.
Treatment
Antibiotic therapy is targeted towards secondary bacterial infection. The most significant opportunists are Mannheimia haemolytica, Pasteurella multocida and Haemophilus somni. Ideally, investigations should be undertaken to guide antibiotic selection. However, in an outbreak situation, antibiotic choice is often empirical.
Under Responsible Use of Medicines in Agriculture Alliance guidelines, fluoroquinolones, third-generation and fourth-generation cephalosporins, and long-acting macrolides should only be used for clinical cases and not preventively. A cost-effective method of selecting cattle for treatment is measurement of rectal temperatures, which should be recorded and rechecked 24 hours later to monitor treatment efficacy, in line with responsible use of antibiotics guidelines.
Duration of treatment is important, but it should be noted recurrence of bacterial infection can occur in up to 25% of cases, requiring repeat antibiotic treatment after 5 to 14 days. This does not indicate antibiotic failure, but reinfection of damaged lungs when antibiotic levels fall below effective concentrations (Scott, 2009).
Supportive therapy involves controlling pyrexia and inflammation in the lungs through administration of NSAIDS or steroids. A reduction in temperature will stimulate appetite and boost demeanour in calves, promoting a more rapid recovery. Dehydration should also be corrected, if present, either by oral or IV fluid therapy. The mechanism of action of clenbuterol is debated, but it is acknowledged to give a clinical benefit when used off licence, IV at 0.5μg/kg to 0.8μg/kg (Nuytten et al, 1986).
Prevention
Vaccination plays a key role in boosting immune status, but it will not prevent disease on its own. Poor biosecurity or overwhelming environmental factors can easily be misdiagnosed by farmers as vaccine failure, reducing their faith in the vaccine and the advice of the vet who prescribed it. Ensuring excellent calf health gives vaccines the optimal chance of success. While closed herds minimise risk of introducing disease, this does not fit every farm business model. Biosecurity – in particular quarantine – must play a key part in disease control for all herds and is often overlooked.
In an ideal world, purchase agreements would be reached between farms with vaccination protocols in place in anticipation of disease associated with the stress of mixing and transportation. In most circumstances, this is impractical or not financially viable.
The Surecalf scheme, piloted in 2007, promotes vaccination prior to sale to reduce the impact of stress and transport on vaccine efficacy. Prevaccinated calves reach premiums of £36 to £57 per 300kg animal (data from calves sold through United Auctions, Scotland in 2008, 2009 and 2012-2014). Unfortunately, from the author’s personal discussion with farmers, a reluctance to increase the outlay for stock, combined with a limited perception of the benefits of such a scheme, appears to exist. Perhaps client education, in particular with regards to the financial benefits of avoiding BRDC outbreaks, could encourage involvement.
Conclusion
BRD is the result of a complex interaction between many factors, including viral and bacterial pathogens; local environment, including ventilation and stocking density; and calf immune status. As such, it can be challenging to treat and can have protracted effects on growth and welfare. As the old adage states, prevention is better than cure.
Fortunately, effective vaccines are available to boost immunity to the primary viral pathogens. While these vaccines are excellent tools, both farmer and vet have responsibility to provide optimum conditions for them to achieve maximum efficacy, by providing appropriate housing and preventing concurrent disease.
For farms where BRDC is an issue, understanding the business is key to discussions about improving accommodation. Where major changes are not possible, don’t be disheartened – look at what you can address at a local level and make a workable plan to take forward.
In this case, 24 Limousin-cross store bullocks, ranging from 13 months of age to 16 months of age, were purchased directly from a dairy farm. The cattle had previously been vaccinated with Rispoval 4 (Zoetis). On arrival they were housed with 300 cattle ranging from 6 months of age to 18 months of age in a single airspace. The cattle already on the unit had been bought as calves and were vaccinated with Rispoval 4.
