12 Apr 2021
Sophie Mahendran runs through protocols to prevent bovine respiratory disease through a combination of management changes, including reducing level of exposure to stressors and infectious agents.

The past decade has seen an evolution in the priorities of farmers, milk buyers and the end consumer, with a real drive and focus towards improving the health and welfare of livestock.
This begins with ensuring the highest standards of care for calves, with a high health status in youngstock helping to build a more robust and resilient farming enterprise. This is mainly due to healthy calves having a shorter time from birth to conception, which is vital due to added rearing costs of £2.26 per day over 24 months (Boulton et al, 2015).
One of the biggest causes of delays to first service is the impact that calf-hood disease has on growth rates, with bovine respiratory disease (BRD) known to be the most serious. Given that puberty is primarily determined by the bodyweight of the heifer, this slowing in growth rates leads to delays in ovarian cyclicity and, therefore, the ability to conceive, with an increased age at first calving by up to six months in calves affected by BRD (Correa et al, 1988).
BRD prevalence ranges between 20% to 78% on UK farms, with associated mortality rates varying between 2% to 8% (Johnson et al, 2017; Mahendran, Booth and Bell, 2017) and one study showing that calves treated for BRD were 2.5 times more likely to die after 90 days (Waltner-Toews et al, 1986).
Longer-term negative impacts of reduced survivability exist to the end of the first lactation in heifers affected by BRD as a calf (Rossini et al, 2004; Bach, 2011).
BRD is a multifactorial disease that has three main areas affecting both the likelihood of infection and the outcome of disease occurrence. These are the underlying health status of the calf itself, the pathogens involved in the infection and the environment that the calf is kept in.
Fundamentally, if we can produce a resilient calf with a strong immune system, housed in an environment that minimises exposure to infectious respiratory pathogens, then we “dilute” the risk posed by BRD in our youngstock.
A multitude of pathogens are involved in BRD infections, with the main ones being bovine respiratory syncytial virus, parainfluenza virus 3, Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, Mycoplasma bovis and infectious bovine rhinotracheitis. Many instances of BRD, especially in younger calves, are thought to be initiated by viral pathogens, with secondary opportunistic bacterial invasion (often from upper respiratory tract commensals; Confer, 2009).
Clinical signs can be very subtle during early stages of the disease, with an increased rectal temperature and depressed demeanour that may manifest itself as a calf having increased lying times (Sutherland et al, 2018) or being slow to get up to feed. This then progresses to include the more typical signs of a cough, increased respiratory rate, and nasal and/or ocular discharge.
This means excellent stockmanship and observation skills are needed to identify affected calves, but studies show that only 54% of farmers are able to correctly identify a calf with BRD (Sivula et al, 1996).
Diagnosis on farm can be made through identification of a combination of these signs, with an increased rectal temperature providing one of the earliest indicators that can be readily detected by staff (Timsit et al, 2011). In outbreak situations, further diagnostics may be appropriate to identify affected calves, with an increasing use of thoracic ultrasound (Figure 1), which has demonstrated a sensitivity of 94% and specificity of 100% in subclinically affected calves (Ollivett et al, 2015).
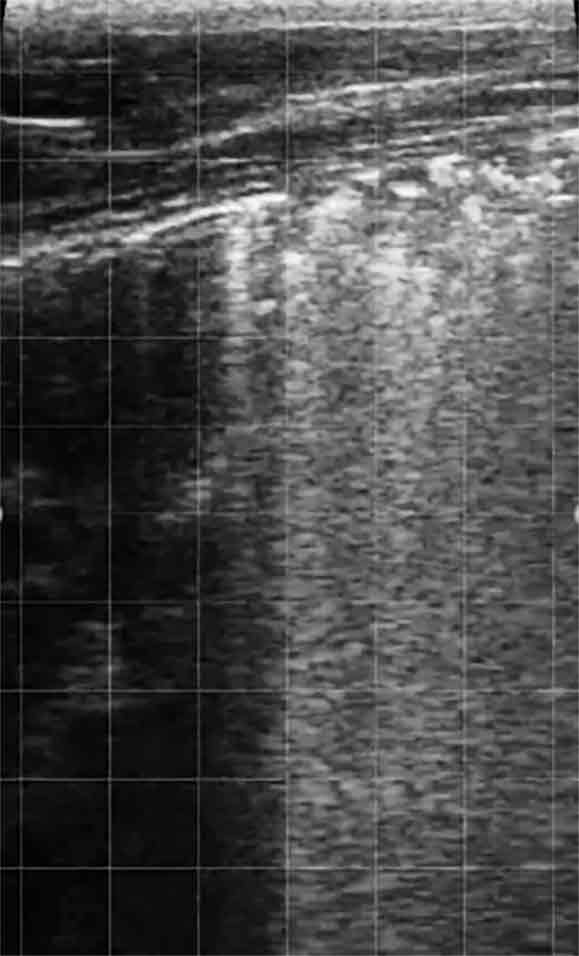
This technique is relatively quick and easy to carry out, making use of a linear rectal probe and spirit on the hair coat to provide good contact. In this way, you can quickly assess calves for signs of pleural inflammation and consolidation, thereby improving prevalence estimates and demonstrating to farm staff calves that have not been correctly identified and treated (which can be helpful as part of staff training).
Once BRD has been identified, appropriate treatment strategies must be in place – preparation of standard operating procedures for farm staff to follow can be beneficial, ensuring both consistency and increased compliance that can improve treatment outcomes. Decisions as to which antimicrobials to use are now being influenced by both European antimicrobial categorisations and milk-buyer stipulations, both of which tend to change on an annual basis.
The new European categorisation system is summarised in Figure 2. Many antimicrobials traditionally used as first-line choices for treating BRD are now in category C (for example, florfenicol; Table 1), with many milk contracts stipulating that macrolides must be reserved as second-line choices.
| Table 1. Examples of antimicrobials used for bovine respiratory disease in European Medicines Agency classes B to D (European Medicines Agency, 2020) | |
|---|---|
| Category | Example antimicrobial |
| B | Fluoroquinolones, third and fourth-generation cephalosporins |
| C | Amoxicillin-clavulanate, florfenicol, macrolides |
| D | Amoxicillin, penicillin, oxytetracycline, trimethoprim/sulfamethoxazole |
This should not result in despair, with many cases of BRD responding well to oxytetracycline – especially with longer-acting formulations available for ease of use. Reports exist of oxytetracycline resistance within the commonly occurring respiratory bacteria (Schönecker et al, 2020), but it is important not to make this assumption. Acquiring samples from either deep nasopharyngeal swabs or bronchoalveolar lavage can allow laboratory culture and sensitivity to identify the presence of resistant bacteria.
Remember, many treatments appear to fail due to insufficient treatment durations, or late detection meaning there is already a lot of lung damage present before treatment has even begun, resulting in perceived treatment failures that, in reality, are disease detection failures.
As well as appropriate antimicrobial therapy, use of NSAIDs forms an important adjunctive treatment, with the aim being to control the inflammatory response in the lungs to reduce development of consolidation. In addition, oxytetracycline has demonstrated some anti-inflammatory properties, meaning it may have a synergistic action when used in combination with an NSAID (Ci et al, 2011).
Published literature has demonstrated variable benefits to NSAID use in calves, with an overall general symptomatic improvement (Deleforge et al, 1994), showing NSAID use is a benefit to calf welfare. Some studies have also specifically shown improved growth rates (Schmidt et al, 2000; Friton et al, 2005), while others have not found the benefits expected from NSAID treatment for BRD (Elitok and Elitok, 2004; Brentnall et al, 2013; Mahendran et al, 2017).
While discussion of best practice treatment protocols is important, reducing the prevalence of BRD on farms should be the long-term aim. The overall “solution” for prevention of BRD must involve a combination of management changes, but all will aim to reduce the level of exposure (“dilution”) that calves have to stressors and infectious agents (“pollution”).
A large part of the “solution” to producing resilient calves is the correct management of colostrum and appropriate nutritional levels during the pre-weaning period. Colostrum provides the calf with its initial supply of globulins (since it is born agammaglobulinaemic), with the immune system slowly starting production in the first few days of life (Bush and Staley, 1980).
Successful passive transfer, therefore, ensures adequate immune protection and is vital for ensuring the health of the calf – calves that receive poor colostrum (either insufficient quality, quantity or not quickly enough) will be at a much higher risk of developing BRD.
Nutrition is the other key factor to ensuring a fully functioning immune system, with sufficient energy and protein required through provision of plentiful supplies of milk to provide the building blocks for production of antibodies and other immune components. Calves that are undernourished (fed less than their energy requirement for maintenance and growth, which is around 8L to 10L per day depending on calf size) will have reduced immune function, with nutritional stress shown to increase cortisol levels and decrease neutrophil function, resulting in a higher risk of developing BRD.
The other “solution” to increasing the resilience of a calf is through the strategic use of vaccinations. Intranasal vaccines provide a fantastic opportunity for provision of early life protection, through both local mucosal antibody responses as well as having a priming effect on the systemic immune system (Woolums, 2007). The vaccines work by inducing both innate immune activation of dendritic and M cells, as well as humoral immunoglobulin A production in the upper respiratory tract mucosa (Holmgren and Czerkinsky, 2005). While vaccination has been shown to reduce the level of physiological lung consolidation during subsequent infections, it also reduces viral shedding, helping to “dilute” the pathogen level in the youngstock housing environment and reducing risk to other calves.
Intranasal vaccines can be given from around 10 days of age, with administration being relatively straightforward when a specially designed vaccine delivery device (vaccine gun) is used. Some have applicator ends that allow the nozzle to be pushed easily into the nostril for optimum delivery of the vaccine (Figure 3).

Use of parenteral vaccines within the first couple of months of life may be less effective due to the presence of maternally derived antibodies (Windeyer and Gamsjäger, 2019), but are again of great benefit for protection during and after the weaning period – especially as parenteral vaccines are available that provide protection against some of the bacterial causes of BRD.
The final area to focus on is the diluting of risk factors that a calf is exposed to. Pathogens involved in BRD infections are numerous, but all are spread via direct calf-to-calf contact, via fomites covered in infectious saliva or nasal secretions or via aerosols. This means if we can dilute the numbers in the environment, we will reduce infection pressures. Isolation of sick calves (if they are kept in groups) will help reduce spread to the rest of the pen, and can help stock people to monitor ill calves more closely.
Minimising the use of feeding equipment for multiple groups will help reduce spread through contaminated teats, but where this is not possible, making sure feeders are used for the most vulnerable, youngest calves first can help (Figure 4). Automatic calf feeders can be a particular source of spread of infectious respiratory pathogens due to a single teat being used to feed large groups – again, the risk can be minimised by at least daily cleaning/disinfection of the shared teat, along with feeding station surfaces that are often covered in calf saliva.

The final management area to optimise is calf housing ventilation – fresh air is vital, with a minimum of eight air changes per hour (Figure 5), at air speeds less than 0.5m/s to avoid causing draughts at calf level (Nordlund, 2008).
This will also help lower levels of dust, which can irritate the respiratory tract and harbour pathogens, as well as noxious gases such as ammonia, which are irritant to respiratory mucosa and can make them more vulnerable to infection.

Calves are the future of the herd, making it imperative to protect their health to ensure future productivity is realised, as well as reducing short-term costs associated with sick calves.
BRD is a performance-limiting disease, and so a multifaceted solution for management is needed to dilute stressors placed on the calf and reduce infectious environmental pollutants that can result in disease.