16 Jul 2018
Sophie Mahendran assesses management options for bovine respiratory disease and how to lower its prevalence among calves.

Figure 1. A fan tube installed to increase air flow into a calf shed.
One of the greatest challenges facing intensive bovine production systems (both beef and dairy) is the occurrence of bovine respiratory disease (BRD) in calves. This disease is tricky to identify, sometimes difficult to treat effectively, and can be very costly – both in terms of labour and equipment – to prevent.
BRD is the second most common cause of calf mortality (following scour/diarrhoea), with strong evidence linking its occurrence with reduced productivity both in the short term, such as growth rates, and the long term due to permanent lung damage, such as future milk production levels in replacement heifers.
In the farming community, the term ”pneumonia” has traditionally been used to describe this disease. However, it is better described as a disease complex due to its mixed aetiology involving multiple pathogens (viral, bacterial and mycoplasma), with multifactorial interactions between the host, pathogen and environment.
It has long been hypothesised respiratory viruses are a primary pathogen, causing direct damage to the lungs’ tissue and clearance mechanisms, as well as facilitating translocation of normal upper respiratory tract bacterial microflora into the lower respiratory tract, allowing establishment of infection in the compromised lung.
The respiratory viruses are also thought to interfere with the calves’ immune system, decreasing its ability to respond to subsequent bacterial infections. The viruses involved in respiratory tract infection are infectious bovine rhinotracheitis (IBR), bovine viral diarrhoea virus (BVDV), bovine respiratory syncytial virus, bovine coronavirus, bovine adenovirus 3 and parainfluenza virus 3 (PI3).
In addition to the viruses, several bacterial pathogens are capable of causing severe damage to the respiratory system: Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma bovis. These pathogens are ubiquitous in cattle and often found in the nasopharyngeal commensal microflora of healthy animals. These bacteria are capable of forming biofilms and have different virulence factors, such as M haemolytica bacteria having lipopolysaccharide, protein adhesin and specific leukotoxins. These enhance the bacteria’s ability to invade and colonise the lower respiratory tract, leading to tissue destruction and an inflammatory response.
Risk factors that appear to predispose calves to development of BRD include transport (specifically, the sorting and loading of cattle), commingling with calves of different ages or calves from different farms, and being sold at auction. It is unclear whether these risk factors lead to increased calf susceptibility and pathogen exposure, or whether they are proxies for poor management practices. However, it is clear stressors are an important factor for development of BRD, and this is thought to be through raised cortisol levels, resulting in alterations in the calf’s immune responses to infection.
The calf’s immune response to BRD infections centres around an initial innate immune response, backed up with an antibody based adaptive immune response. The respiratory tract has several physiological adaptations to aid in pathogen trapping and removal, such as nostril hairs providing a physical barrier to large particles, a mucous lining of the respiratory tract to help trap pathogens and that contains several antimicrobial factors such as lysozymes and IgA, along with cilia that help transfer particulates back along the respiratory tract towards the nares for expulsion.
Non-specific immune cells also have a vital role, with bronchoalveolar macrophages and neutrophils having phagocytic functions elicited on detection of pathogen associated molecular patterns. This, in turn, can induce release of cytokines (proteins excreted by leukocytes to modulate the immune system), such as interleukin 8, which act as chemoattractants for immune cells to sites of inflammation.
Some respiratory viruses, such as IBR and PI3, are able to infect the ciliated respiratory epithelium, as well as respiratory immune cells, so interfering with their function of bacterial clearance and killing, resulting in increased chances for bacterial infection. In addition to the purely respiratory pathogens, prior infection with BVDV can create a local and systemic immunosuppression through infection of mononuclear phagocytes, again increasing the risk of further pathogen establishment.
Successful treatment of BRD relies on early identification, prompt application of treatments, and careful monitoring to assess disease progression and the requirement for further treatments. Early identification of affected calves can be improved through stockman training and use of tools.
Treatment of BRD generally comprises of combined antimicrobial and NSAID drugs. Successful use of diagnostics to identify pathogens, along with establishing antimicrobial sensitivity, can be challenging and expensive with BRD, but should definitely be used in high prevalence or high mortality situations.
A plethora of antimicrobials are available for the treatment of BRD, many of which are listed in Table 1 and NSAIDs listed in Table 2.
| Table 1. Antimicrobials licensed for treatment of respiratory disease in calves in the UK. The red boxes indicate critically important antimicrobials, which should be used under appropriate culture and sensitivity diagnostics | ||
|---|---|---|
| Active compound | Antimicrobial drug name | Recommended dose rate |
| Amoxicillin | Amoxypen, Betamox, Duphamox | 7mg/kg given for up to 5 days |
| Vetrimoxin LA | 15mg/kg for 3 to 5 days | |
| Bimoxyl LA | 15m/kg, can be repeated after 48 hours | |
| Amoxicillin clavulanate | Synulox RTU, Noroclav, Combiclav | 8.75mg/kg for up to 3 to 5 days |
| Amoxicillin trihydrate | Clamoxyl RTU | 7mg/kg for up to 5 days |
| Ampicillin | Amfipen | 15mg/kg given twice 48 hours apart |
| Cefalexin | Ceporex | 7mg/kg for up to 5 days |
| Cefquinome | Cobactan | 1mg/kg for up to 3 to 5 days |
| Ceftiofur | Readycef | 3mg/kg for 3 days |
| Excenel, Ceftiocyl, Eficur, Cevaxel RTU, Cemay | 1mg/kg for up to 5 days | |
| Ceftiofur and ketoprofen | Curacef | 1mg/kg ceftiofur and 3mg/kg ketoprofen for up to 5 days |
| Danofloxacin | Advocin | 1.25mg/kg for 3 days |
| Enrofloxacin | Enroxil, Fenoflox, Baytril | 5mg/kg for up to 3 to 5 days |
| Florfenicol | Fenflor, Shotaflor, Nuflor, Selectan, Florkem | 20mg/kg given twice 48 hours apart |
| Norfenicol | Single dose of 40mg/kg | |
| Kefloril | 15mg/kg given twice 48 hours apart | |
| Florfenicol and flunixin meglumine | Resflor | Single dose: 40mg/kg for florfenicol, 2.2mg/kg for flunixin |
| Gamithromycin | Zactran | Single dose of 6mg/kg |
| Marbofloxacin | Quiflor, Marbocyl | 2mg/kg for up to 3 to 5 days |
| Marbonor | Single dose of 2mg/kg | |
| Marbox | Single dose of 8mg/kg | |
| Forcyl | Single dose of 10mg/kg | |
| Oxytetracycline | Vetroxy LA, Tetroxy Vet, Terramycin LA | Single dose of 20mg/kg |
| Engemycin 10% | 10mg/kg to 20mg/kg given twice 48 hours apart | |
| Alamycin LA | Single dose of 30mg/kg | |
| Alamycin | 1.5mg/kg to 4mg/kg for 3 to 5 days | |
| Oxytetracycline and flunixin | Hexasol LA | Single dose 30mg/kg of oxytet, 2mg/kg of flunixin |
| Procaine benzypenicillin | Depocillin | 12mg/kg for up to 5 days |
| Procaine penicillin | Norocillin | 10mg/kg for 3 to 5 days |
| Procaine penicillin and dihydrostreptomycin sulphate | Pen and Strep | 8mg/kg penicillin, 10mg/kg dihydrostreptomycin |
| Spectinomycin | Spectam | 20mg/kg to 30mg/kg for up to 5 days |
| Sulfadiazine and trimethoprim | Tribrissen | 15mg/kg to 22.5mg/kg for up to 5 days |
| Duphatrim IS, Bimotrim Co | 15mg/kg for up to 5 days | |
| Tildipirosin | Zuprevo | Single dose of 4mg/kg |
| Tilmicosin | Tilmovet | 12.5mg/kg for 3 to 5 days |
| Apotil, Milbotyl, Micotil | Single dose of 10mg/kg | |
| Tulathromycin | Draxxin | Single dose of 2.5mg/kg |
| Tylosin | Tylucyl, Bilovet, Tylan 200, Pharmasin | 5mg/kg to 10mg/kg for 3 days |
| This table is intended as a reference guide only and is not necessarily exhaustive – check individual datasheets before choosing or applying a product to a patient. Information is taken from the online NOAH Compendium and the VMD website. It is recommended to check packaging or data sheets as licensing indications are changing rapidly and products are frequently coming to market. | ||
| Table 2. NSAIDs licensed for treatment of respiratory disease in calves in the UK | |
|---|---|
| Active compound | Dose |
| Carprofen | 1.4mg/kg by single SC injection |
| Flunixin meglumine | 2.2mg/kg for up to 5 days |
| Meloxicam | Single dose of 0.5mg/kg |
| Ketoprofen | 3mg/kg for up to 3 days |
| Tolfenamic acid | 2mg/kg given twice 48 hours apart |
| This table is intended as a reference guide only and is not exhaustive – check individual datasheets before choosing or applying a product to a patient. Information is taken from the online NOAH Compendium and the VMD website. It is recommended to check packaging or data sheets as licensing indications are changing rapidly and products are frequently coming to market. | |
Around 20 different active compounds are licensed, often resulting in the decision on which one to use being heavily influenced by price and duration of action.
Single-dose treatments are often desirable due to the reduced time and effort required for application of a complete course, which can be a very important factor for busy stock people. However, little research exists into the most effective duration of treatments.
In terms of the most efficacious antimicrobial treatment to use, again a lack of clear research exists comparing all available actives, and their pharmacokinetics (movement and distribution of drugs within the body) and pharmacodynamics (relation between drug concentration and antimicrobial activity).
Drug guidelines are based on pharmacokinetic/dynamic models based on plasma concentrations of the drug; however, this does not fully take into account the drug levels found in lung tissue, which is important for treating respiratory disease. An example of this is macrolides, which have low plasma levels compared to their minimum inhibitory concentrations (MIC; the lowest concentration of drug that inhibits bacterial growth), but are able to concentrate in the pulmonary epithelial lining fluid in the lungs, therefore providing good treatment for respiratory infections.
The pressure to use antibiotics in a responsible and evidence-based manner is increasing, with the added challenge of ensuring farmers are also complying with these requirements as they are generally the providers of first-line treatments. Vets should be encouraged to provide standard operating procedures for treatment of BRD to help ensure consistency across all farm personnel, as well as allowing true identification of treatments that do not appear to be efficacious on farm. The most appropriate antimicrobial and NSAID should be selected on a farm-by-farm basis, often with florfenicols and macrolides proving popular choices with vets in the UK.
Correct management of the housing environment can be one of the most important factors for long-term reductions in BRD prevalence, but they often require the greatest cost investment. Redesigning of sheds to improve ventilation, reduce disease transmission, and raising hygiene levels requires careful planning and consideration to ensure efforts are not wasted and expectations do not fall short.
One of the most common areas for improvement is the ventilation of the calf shed, with the overarching aim being to improve air quality. This can be achieved by increasing air inlet area in the sides of the shed or through the use of positive pressure ventilation systems, such as tube fans, to blow air into the shed (Figure 1).

Installation of tube fans should only be done following consultation and specific design of a tube with a qualified agricultural engineer who can assess correct tube placement, the size of the fan required and the correct placement of the holes within the tube to ensure consistent air delivery throughout the whole shed.
While tube fans are able to have a significant impact on air quality by reducing bacterial numbers and ammonia levels, it must be remembered some components of air quality are highly dependent on the outside air, especially humidity levels and temperature. Humidity can have a big impact on respiratory health in calves and adult cattle, with humidity levels recommended to be less than 70% (Figure 2).
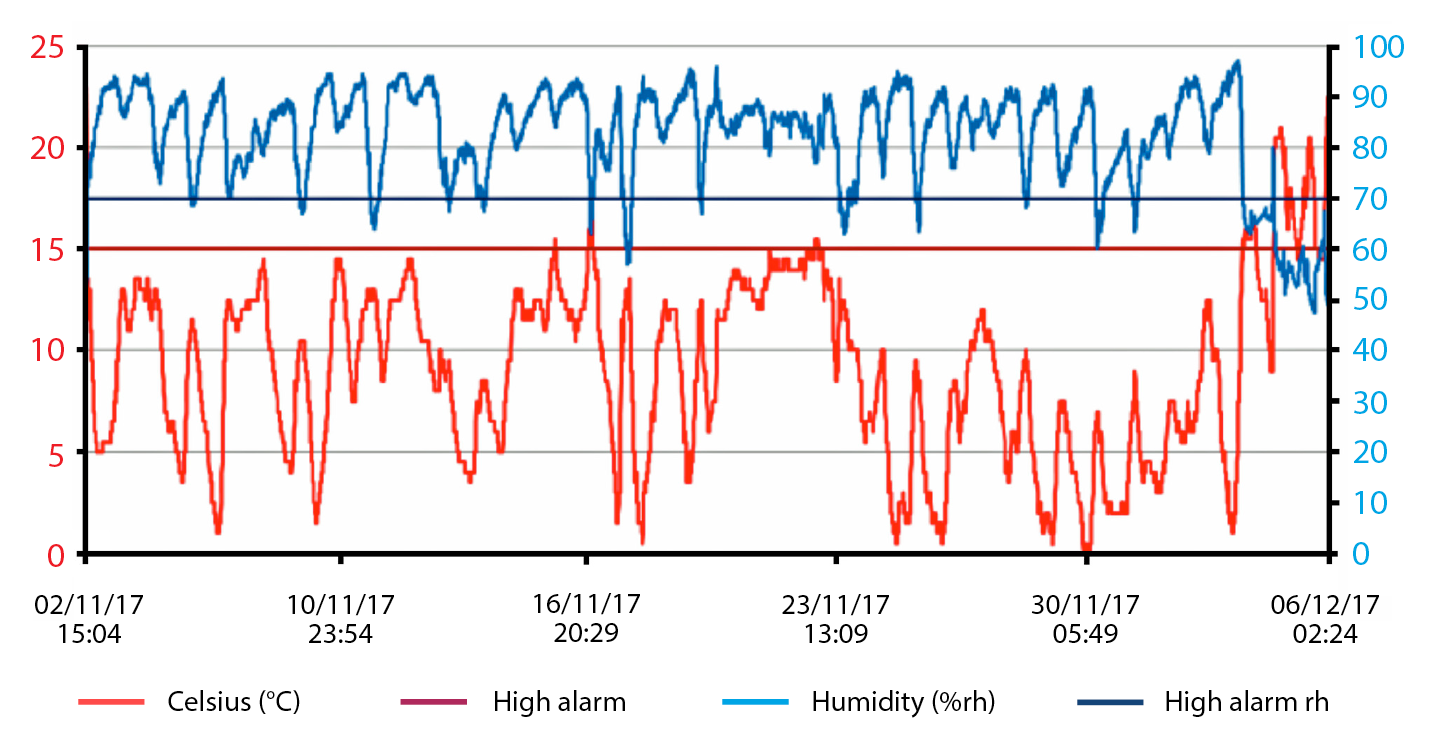
However, the UK weather in the late autumn, winter and early spring means humidity levels are often quite high, making it harder to maintain, through ventilation alone, low humidity levels in sheds containing livestock. This makes shed hygiene critical, with appropriate drainage for urine and milk, and maintaining clean and dry bedding helping to improve the air quality at calf level.
Alternatively, negating the need to redevelop shed space, investment into calf hutches is another option. These housing modules have been in use for more than a decade and require a large initial investment, but can provide very rapid improvements in calf heath – both for diarrhoea and BRD. While the isolation of calves very effectively reduces the spread of pathogens, increasing research has taken place into the importance of social contact for calves with their cohort, as well as potential issues with temperature control in these hutches during extremes in weather conditions.
It is also worth remembering hutches are very much a one trick pony – they are great for housing calves, but if no longer required, have no other useful function on farm, whereas a shed can be repurposed for storage or housing other animals.
Although not discussed in detail here, use of vaccination against respiratory disease is another component of preventive strategies that can be effectively used to reduce occurrence of clinical disease. Many products are available, each conferring immunity to different pathogens and with different application programmes. Again, care should be taken to select vaccines on a farm-by-farm basis, taking into account the risk period for those calves and trying to ensure vaccines are given prior to infectious challenge to have the greatest impact on disease prevention.
BRD is one of the trickiest herd level problems practitioners have to deal with on a regular basis. Having an understanding of some of the predisposing immunological and environmental factors that contribute towards this disease, along with knowledge of available treatments and further preventive actions, can help in the development of successful farm protocols and a reduction in BRD prevalence.
Ensuring the best health of calves early in their lives enables maintenance of target growth rates and better future health, allowing development of more productive animals long term.