6 Jun 2019
BVDzero Award finalist: strategies to convince a farmer to eradicate BVD
CASE STUDY: 2016 BVDzero awards finalist Angela Damaso explains how limited resources, farmer apathy, biosecurity challenges and various other issues resulted in an incomplete BVD eradication programme.

Photo © ellisia / Fotolia.
Background
This entry examined herds of cross-bred British Friesian and Jersey cows belonging to a Welsh agricultural college. The herds block calve in spring and autumn and had recently increased in size from 150 animals to 250 in 2016. Latterly, the herd has been run as a closed herd, with the last stock – 30 cows from Dumfriesshire – being brought in in 2009.
Both herds graze on pasture in good weather in separate fields, and are housed under the same roof in the winter. Due to the high prevalence of bovine tuberculosis (bTB) in the region, the farm has been under movement restrictions on and off and, at the time of writing, was down with the disease.
BVD on the farm

In 2011, poor production and fertility performance led to suspicion of bovine viral diarrhoea (BVD). Although the herd had been vaccinated against BVD, the farmer commented that the protocol was not thoroughly followed, due to lack of engagement from the staff in completing the two injection primary course.
An on-farm BVD investigation was initiated using both a bulk milk virus and a check test (serology in 10 calves from 9 months up to 15 months of age) in each herd. All results came back positive, revealing the virus was circulating in the two herds, and an eradication program commenced on the farm.
The strategy used to eradicate BVD in this farm was by means of elimination of persistently infected (PI) animals only, and vaccination was not pursued. All adult cattle and youngstock over 30 days old were individually blood sampled, and samples were then pooled and tested at the receiving laboratory. Seven of the pools were positive and the animals within these pools were resampled and retested three to four weeks later. A second positive result indicated persistent viraemia and these animals were interpreted as persistently infected (PI) and culled; however, in some cases, culling occurred as late as eight months post-confirmation.
All newborns over 30 days of age were tested until April 2013, and due to the negative results at that time, the farmer decided to stop testing and the eradication program ceased.
In August 2013, as a consequence of the farm expansion, a management decision was made to shift the youngstock rearing to a contractor, after the end of the bTB restrictions.
BVD control then switched to a monitoring-based system, where the herds were checked quarterly. In 2015, this revealed the presence of BVD but the farm manager questioned the reasons behind the recent outbreak and whether it was cost-beneficial to carry out a new PI hunt, which led to an impasse in the investigation.
Discussion
There were several issues compounding the BVD challenge on-farm:
- BVDV PCR positive on bulk milk tank two years after eradication program ceased
- High biosecurity and biocontainment challenges on-farm
- Lack of motivation from farm manager to carry out new cycle of disease control and eradication
- PI animals not removed immediately upon confirmation
- Testing of newborn calves ceased too early in the battle to control BVD
- Absence of vaccination
- Moving youngstock to be reared away
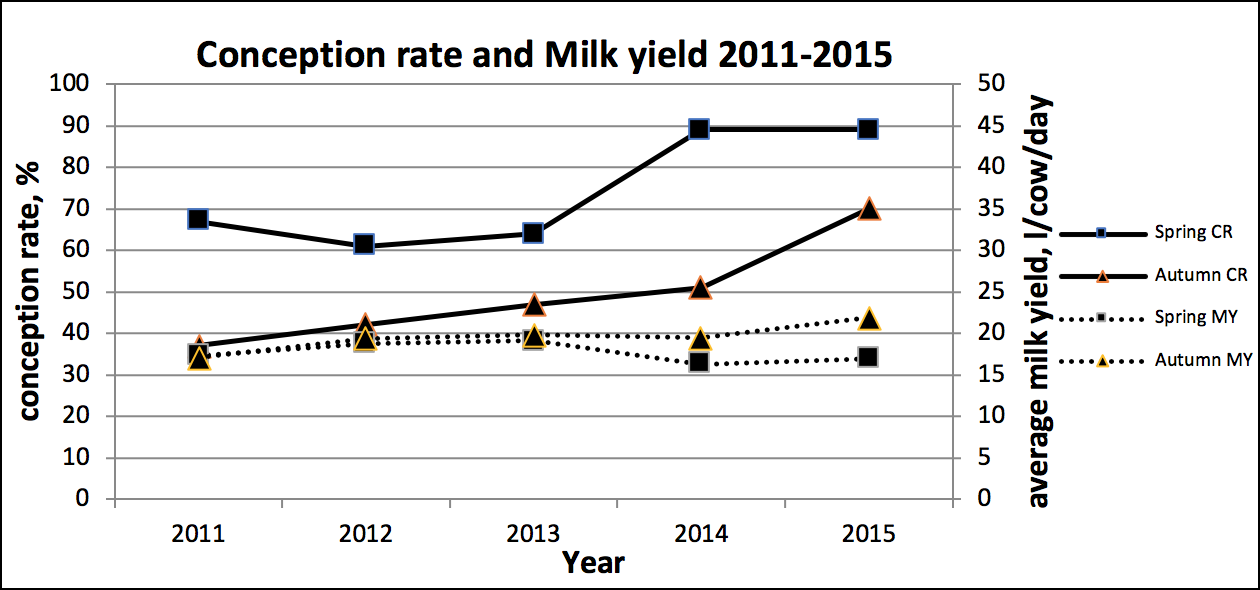
Data analysis
In order to compel the farm team to strive to control BVD, analysis of conception rate and milk yield took place. This showed improvements, over time, for both herds (albeit with a blip for the spring herd in 2014, due to other factors).
Further analysis showed an improvement in age at first calving, when BVD was removed from the herd.
Follow up and conclusion
Despite highlighting shortcomings in biosecurity to the team, farm practices regarding biosecurity remain unchanged, with the farm manager claiming limited resources.
As a result of this stalemate, a thorough vaccination protocol was put in place for the bulling heifers with a one-shot live BVD vaccine, with the aim of preventing new PIs being born. All newborn calves were then tested for 12 months following the removal of the last PI from the farm.
This incomplete eradication programme had its own shortcomings, with one PI never being tested and surviving through to the next rearing and calving period.
The key measure of herd fertility performance for such block calving herds was age at first calving and holding to service data. These depend largely on a successful calf rearing programme. BVD is a disease likely to impair calf growth, as it predisposes animals to health problems – such as neonatal diarrhoea syndrome and bovine respiratory disease, among other diseases – with subsequent impact on herd health.

Bovela lyophilisate and solvent for suspension for injection for cattle contains modified live BVDV-1, non-cytopathic parent strain KE-9: 104.0–106.0 TCID50, Modified live BVDV-2, non-cytopathic parent strain NY-93: 104.0– 106.0 TCID50. UK: POM-V. Further information available in the SPC or from Boehringer Ingelheim Animal Health UK Ltd, RG12 8YS, UK. Tel: 01344 746960 (sales) or 01344 746957 (technical). Email: [email protected]. Bovela is a registered trademark of Boehringer Ingelheim Vetmedica GmbH, used under licence. ©2019 Boehringer Ingelheim Animal Health UK Ltd. All rights reserved. Date of preparation: Apr 2019. AHD12279. Use Medicines Responsibly.
