11 Apr 2023
Communicating scour messages to farming clients
Ginny Sherwin covers work around scour and how to communicate to clients on when to intervene, and what to look out for, putting a practical spin on many of the complex areas around scour prevention, management and treatment.
Neonatal calf mortality (defined as one day of age to weaning; Compton et al, 2017) has been reported to be 6% for dairy calves and 2.86% for beef calves in the UK, and has remained fairly static over the past decade.
This has been estimated to cost the industry £11.6 million per annum (Hyde et al, 2020).
One of the major contributors to neonatal mortality in calves is the prevalence of calf diarrhoea, colloquially referred to as scours; this represents an area where vets can have a significant input into on farm.
Why do calves die from neonatal diarrhoea?
Calves most commonly die from neonatal diarrhoea due to dehydration, with the replacement of lost fluids, plus providing the maintenance fluid requirements of a calf being vital to ensuring its successful outcome. The maintenance requirement for water for calves is 250ml per 24 hours (6L in 24 hours), which can come from milk/commercial milk replacer and free access water. The estimation of the volume of fluid required in a calf is based on clinical signs, which estimate the proportion of fluid lost by the calf; for example, a loss of 5% bodyweight for a 40kg is 2L.
Replacement of fluids can be delivered orally or by IV fluid therapy, depending on the severity of the clinical signs.
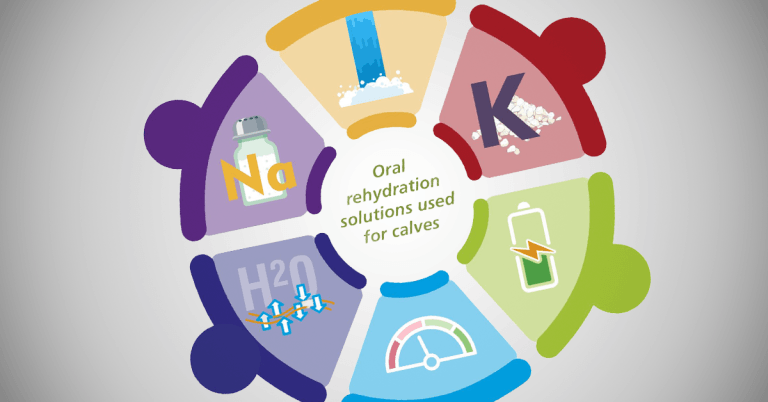
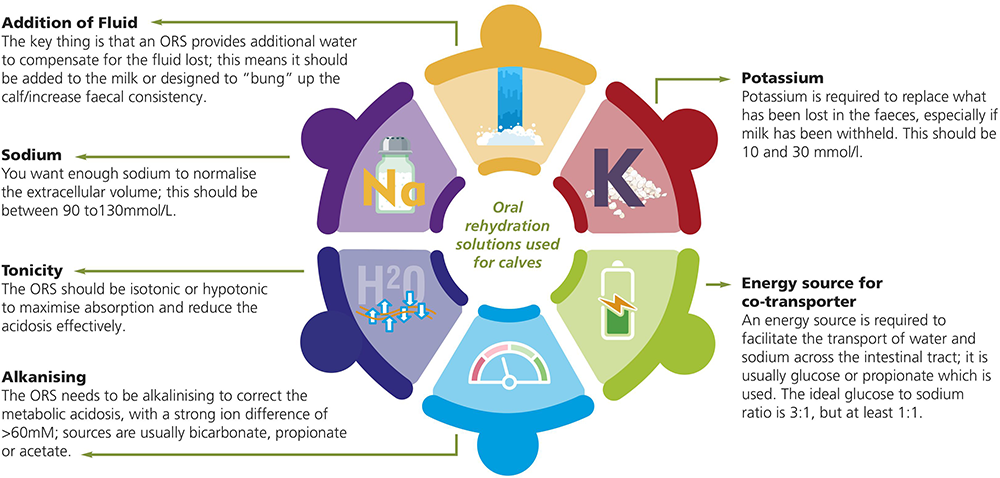
Numerous different commercial oral rehydration solutions (ORS) are available – a summary of some of the key areas to look at when choosing a product are highlighted in Figure 1.
The other reason why calves die from neonatal diarrhoea is due to damage of the gastrointestinal tract lining, which allows for translocation of the bacteria from the intestines into the bloodstream. The inflammatory response is also the reason for infectious causes of diarrhoea to result in pyrexia. The use of NSAIDs is important for reducing the intestinal damage, caused by the response of the immune system to the presence of an antigen. A single injection of meloxicam was reported to also increase consumption of milk, solid feed and water, which has been hypothesised to be related to controlling the pyrexia (Todd et al, 2010).
Depending on the severity of the diarrhoea and the pathogens present, antibiotics may be required to prevent septicaemia due to secondary translocation of bacteria into the bloodstream.
Isolation of sick calves, either as individuals or as a group, with disinfection between pens/calves of both boots and waterproofs, as well as shared equipment, will aid in reducing the transmission on the farm.
Why is neonatal diarrhoea a problem?
The risk factors for calf diarrhoea can be broadly categorised into two topics: colostrum management and exposure to the pathogen.
From a practitioner’s perspective, it is important to determine where the risk is to implement a successful prevention plan. It is worth noting that farms exist with both failure of passive transfer (FPT) and pathogen exposure issues, requiring changes being implemented in both areas.
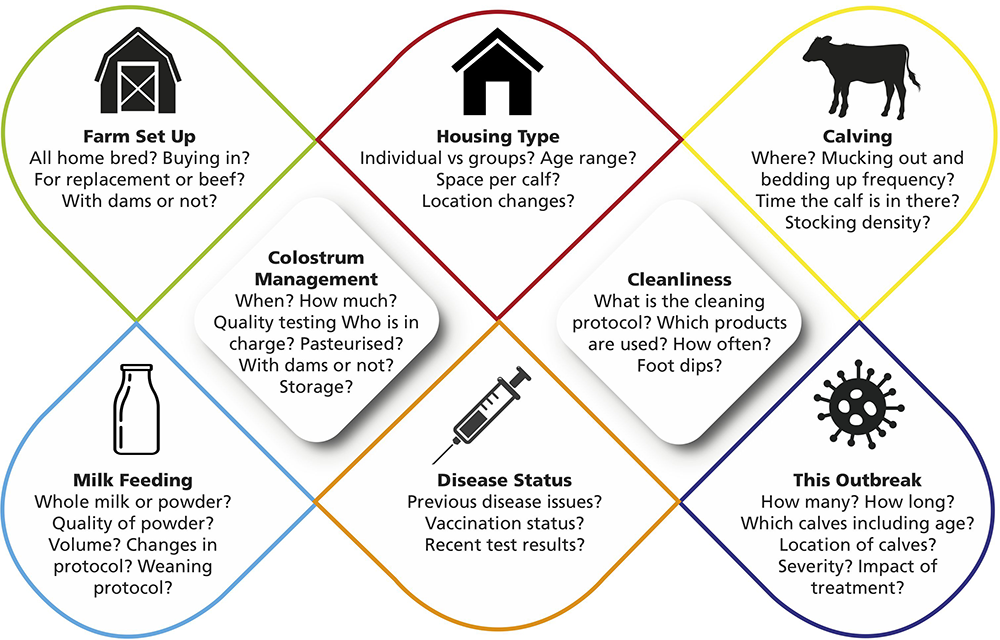
Taking a detailed history of the farm management and calf management is key in aiding determination of where the issues resulting in the outbreak of neonatal diarrhoea exist. Some of the main areas to focus on are shown in Figure 2.
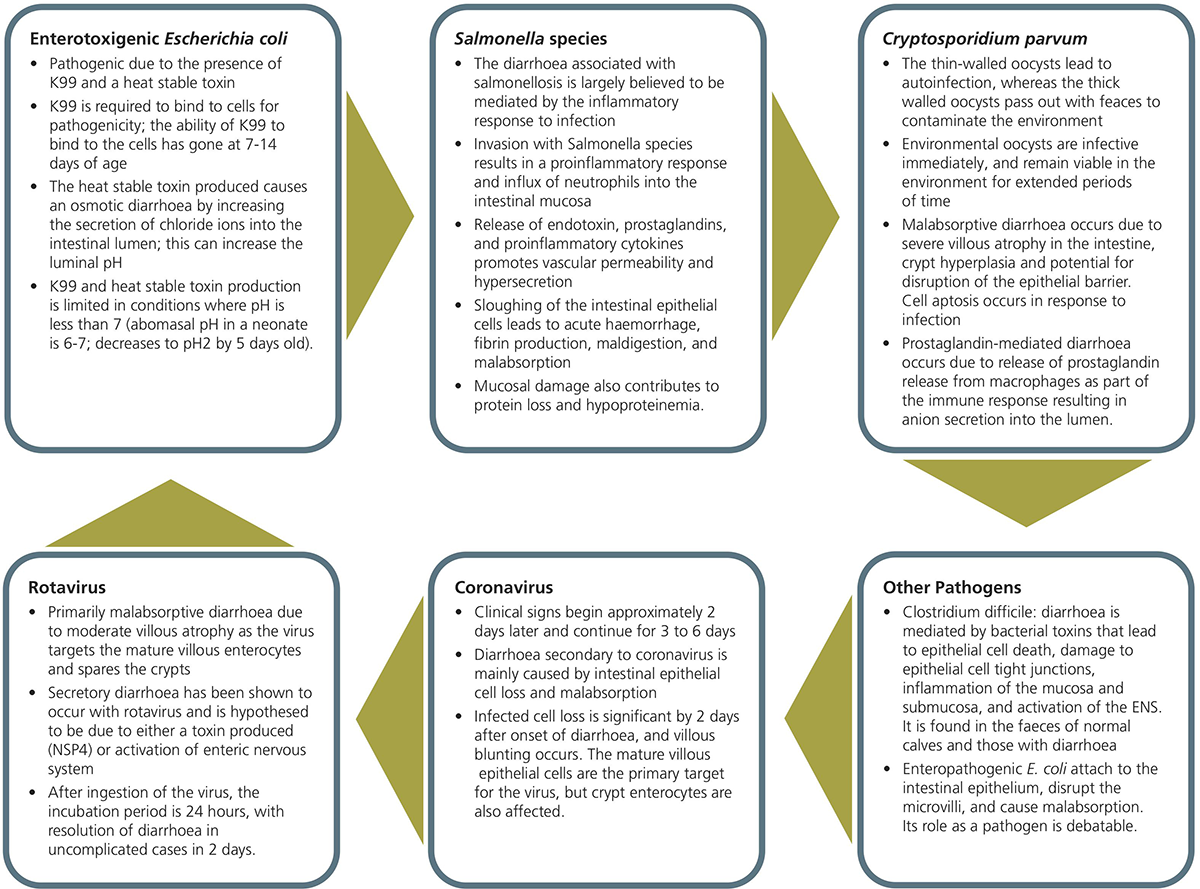
One of the main prevention strategies is separating the calf from the source of infection, which can be faeces, both from adult cows in the calving yard, and also from other calves. Multiple different pathogens are involved with neonatal diarrhoea, which are summarised in Figure 3, with the cause of an outbreak frequently involving more than one pathogen.
Another aspect associated with calf diarrhoea is cleaning and disinfection of both the housing environment and also feeding equipment. Ensuring that equipment and housing is cleaned with hot water (more than 60°C), and a disinfectant that is active against pathogens such as Cryptosporidium (including the use of the correct time) is key to reducing the environmental infection pressure.
One of the most obvious and critical focus points is around colostrum management and feeding, with approximately between one in five dairy calves and one in seven beef suckler calves reported to have FPT (MacFarlane et al, 2015; Haggerty et al, 2021; Bragg et al, 2020). Therefore, it is essential that colostrum management is assessed and a protocol put in place to ensure optimal transfer of colostral antibodies. This includes:
- Volume – recommended to feed 10% to 15% bodyweight in the first feed to ensure that the critical cut off 150g of IgG is consumed. This is important as research has highlighted that feeding 4L of colostrum instead of 2L significantly increased daily liveweight gain (1.03kg/day versus 0.80kg/day; DLWG), increased survival rate to second lactation (87.1% versus 75.7%), and increased milk yield in first and second lactation (17,042L versus 16,015L; Faber et al, 2005).
- Quality – the quality of the colostrum ensures that an adequate concentration of IgG is present in the colostrum fed, with 22% being the cut-off for 50g/L of IgG. Quality of colostrum has been reported to vary between different farms, parity of cows and breeds.
- Timing – an increase in intestinal wall permeability occurs at birth to facilitate the absorption of IgG, which occurs through a non-selective macromolecular transport system (Staley et al, 1972). This increased permeability decreases over time and has disappeared by 24 to 36 hours of life (Besser et al, 1985). This window of increased intestinal absorption has been reported to shorten after colostrum intake (Stott et al, 1979), making the volume and quality received in the first feed even more important. The recommendation is that calves receive colostrum within the first two hours of life, but a six-hour target may be more realistic, as well as being a legal requirement (Defra, 2003).
- Cleanliness – colostrum quality is impacted by cleanliness, as the IgG in the colostrum will bind any pathogens that are present in the colostrum, therefore making them unavailable for absorption into the bloodstream. Research has reported that approximately 25% colostrum samples fed to dairy calves are classified as dirty (total bacterial count more than 100,000cfu/ml).
How can we improve colostrum management?
Increasing the amount of colostral antibody transferred to the calf has been reported to have an impact on DLWG in calves (Bragg et al, 2023). Colostrum quality can be boosted to faecal pathogens through the vaccination of the dam during the dry period, to promote the production of antibodies against rotavirus, Escherichia coli and coronavirus.
Vaccination of the dam is required, as the vaccines have limited use in calves during the risk period (less than three weeks of age) because of their immature immune system and the interference of maternally derived antibodies (Chase et al, 2008; Cortese, 2009).
One study reported that calves from vaccinated dams had significantly higher passive antibody titres than those born to non-vaccinated dams (Hodgins and Shewen, 1996), which was associated with reductions in both the severity of diarrhoea and the duration of shedding.
We also need to determine whether the colostrum management is successful, in terms of quantifying the prevalence of failure of passive transfer. This requires blood sampling healthy calves that are two to seven days old (avoid dehydrated calves as they can give false positive results) and determining the serum IgG results.
Different cut-offs have been proposed for both complete and partial FPT; however, commonly, 8.4% Brix is used (or 55mg/dL). The key thing is that this must be reported back to the farm, as it can be used as talking point or as a motivator for farm staff to keep going with proactive colostrum management. This can be via a whiteboard, WhatsApp group, sit-down meetings or something similar.
A review of using data relating to youngstock herd health, including diarrhoea, can be found in Sherwin et al (2016).
What does success look like?
The success in managing outbreak of diarrhoea is often dependent on the success of the communication around implementing change on the farm.
This can often be a difficult conversation as it relates to issues in mismanagement of the calves by farm staff, who are commonly working long hours already and are acutely aware of what the issues are.
Success with these discussions involves being prepared and having a plan, providing a key focus area for the farm staff to concentrate on, which is realistic and has measurable outcomes. The farm staff need to buy into the recommendations, and this may involve some compromises on any initial plans to ensure compliance on farm, with a review of the progress set at a time point.
A fundamental part of a preventive approach is the continual measuring and monitoring to determine the impact of any changes implemented on farm.
- Diarrhoea outbreaks in calves are often due to multiple pathogens (including zoonotic pathogens), involving multiple risk factors, so a holistic approach is required.
- Colostrum management is key to boosting a naive and immature immune response.
- Colostrum quality can be boosted through ensuring good dry cow management and vaccination to diarrhoea-specific pathogens.
- Cleanliness is important to prevent the spread of disease, with detailed protocols required around how, with what and when.
- Measuring and monitoring allows for the impact of preventive measures to be determined.
- This article was supplied by Boehringer Ingelheim as part of the company’s educational programme following the launch of Fencovis.
References
- Besser TE (1985). Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves, J Dairy Sci 68(8): 2,033–2,037.
- Bragg R et al (2020). Prevalence and risk factors associated with failure of transfer of passive immunity in spring born beef suckler calves in Great Britain, Prev Vet Med 181: 105059.
- Bragg R et al (2023). Effect of neonatal immunoglobulin status on the outcomes of spring-born suckler calves, Vet Rec 192(6): e2587.
- Chase CCL et al (2008). Neonatal immune development in the calf and its impact on vaccine response, Vet Clin North Am Food Anim Pract 24(1): 87-104.
- Compton CWR et al (2017). Invited review: a systematic literature review and meta-analysis of mortality and culling in dairy cattle, J Dairy Sci 100(1): 1-16.
- Cortese VS (2009). Neonatal immunology, Vet Clin North Am Food Anim Pract 25(1): 221–227.
- Defra (2003). Guidance: Caring for beef cattle and dairy cows.
- Faber SN et al (2005). Case study: effects of colostrum ingestion on lactational performance, Prof Anim Sci 21(5): 420-425.
- Haggerty A et al (2021). Risk factors for poor colostrum quality and failure of passive transfer in Scottish dairy calves, J Dairy Res 88(3): 337–342.
- Hodgins DC and Shewen PE (1996). Preparturient vaccination to enhance passive immunity to the capsular polysaccharide of Pasteurella haemolytica A1, Vet Immunol and Immunopathol 50(1-2): 67-77.
- Hyde RM et al (2020). Quantitative analysis of calf mortality in Great Britain, J Dairy Sci 103(3): 2,615-2,623.
- MacFarlane JA et al (2015). Identification and quantification of factors affecting neonatal immunological transfer in dairy calves in the UK, Vet Rec 176(24): 625.
- Sherwin V et al (2016). Measuring health and performance in preweaning dairy calves, In Pract 38(3) 113-122.
- Staley TE (1972). The ultrastructure of neonatal calf intestine and absorption of heterologous proteins, Anat Rec 172(3): 559-579.
- Stott GH et al (1979). Colostral immunoglobulin transfer in calves I. Period of absorption, J Dairy Sci 62(10): 1,632-1,638.
- Todd CG et al (2010). Nonsteroidal anti-inflammatory drug therapy for neonatal calf diarrhea complex: effects on calf performance, J Anim Sci 88(6): 2,019-2,028.
Meet the authors
Virginia Sherwin
Job Title