13 Mar 2017
Ectoparasites in dairy cattle: summer grazing precautions
Andrew Forbes discusses parasitic and pest species that can impact on the grazing season and how best to control the problem.

Figure 1. Chillingham cow with face flies.
Pest flies, midges and ticks can be a source of annoyance to cattle at grass and, more importantly, can act as vectors for a number of infectious diseases. Behavioural responses to fly attack can be marked, but do not necessarily result in production losses as hosts can compensate for such disruptions to ensure feed intake and, hence productivity, is only affected to a limited extent.
On the other hand, some of the vector-borne diseases, such as redwater, are not only serious, but potentially fatal. The only broad spectrum insecticides licensed for cattle are pyrethroids, so their use must be responsible to limit the inevitable selection for resistance and should ideally be used within an integrated parasite/pest management approach, which includes non-chemical methods. If nuisance fly control is focused on the prevention of pinkeye and summer mastitis from July to September, this should also offer some tangential control of other ectoparasites simultaneously.
Mechanical control in dairies – for example, high-speed fans and fly zappers – can augment fly control measures taken at pasture. The aims of ectoparasite control in cattle at grass are to limit parasite/pest populations and minimise the risks of vector-borne disease.
During the grazing season, particularly in the warmer months from July to September, cattle can play host to several parasitic and pest arthropod species, collectively referred to as ectoparasites.
The impact of these infestations derives from their direct adverse effects on the host, behavioural responses and their role as vectors of numerous viral, bacterial and nematode pathogens. In addition, treatment and control costs need to be included to assess the full economic impact of ectoparasites.
However, the UK can be considered to be relatively lucky insofar as, in terms of economic impact, of the top-ranked arthropod infestations of cattle (Steelman, 1976) only horn and stable flies are found in the UK, where they appear to be less troublesome compared to other regions. The list is:
- tsetse fly – Glossina species
- tropical bot fly – Dermatobia hominis
- warble fly – Hypoderma species
- horn fly – Haematobia irritans
- stable fly – Stomoxys calcitrans
It should also be noted, since this assessment was conducted, hypodermosis was eliminated in the 1990s as an endemic disease in the UK (Webster et al, 1997).
Flies are common on most dairy farms in the UK, although the prevalence of different species varies regionally (Hillerton et al, 1984; Titchener et al, 1981). Other parasites that can affect pastured dairy cattle include midges (Culicoides species), which are vectors of bluetongue and Schmallenberg virus, and ticks (Ixodes ricinus), which transmit louping ill virus, Anaplasma phagocytophilum (tick-borne fever) and Babesia divergens (redwater).
Summary information on four of the most important species of nuisance and biting flies, midges and ticks are provided in Table 1. This article focuses on the biology of pest and biting flies and their control.
| Table 1. Summary information on common ectoparasites found associated with grazing cattle in the UK | |||||||
|---|---|---|---|---|---|---|---|
| Species | Common name | Breeding | Adult feeding | Effect on growth | Effect on milk yield | Vector of | Disease |
| Musca autumnalis | Face fly | Fresh cattle dung | Ocular and nasal secretions; skin lesions | No | No | Moraxella bovis | Infectious bovine keratoconjunctivitis |
| Thelazia species | Parasitic conjunctivitis | ||||||
| Parafilaria bovicola | Parafilariasis | ||||||
| Hydrotaea irritans | Head fly | Dung, manure, carrion, rotting vegetation | Ocular and nasal secretions; skin lesions | No | No | Archanobacterium pyogenes | Summer mastitis |
| Stomoxys calcitrans | Stable fly | Manure, bedding | Blood | Yes | Yes | ||
| Haematobia irritans | Horn fly | Fresh cattle dung | Blood | Yes | Yes | Staphylococcus aureus | Mastitis |
| Culicoides species | Midge | Manure | Blood | No | No | Bluetongue virus | Bluetongue |
| Schmallenberg virus | Abortion | ||||||
| Ixodes ricinus | Sheep tick | On host and in vegetation mat | Blood | No | No | Louping ill virus | Louping ill |
| Anaplasma phagocytophilum | Tick-borne fever | ||||||
| Babesia divergens | Redwater | ||||||
Nuisance flies
As a general rule, nuisance flies have limited direct effects on either lactating dairy cows or young heifers in terms of productivity (Schmidtmann et al, 1981; 1984), despite showing some disruptive behaviour (Schmidtmann and Valla, 1982).
Behavioural responses to nuisance flies that, at first sight, appear to be detrimental to performance can have no effect, or the opposite. An example of this is in face flies, Musca autumnalis (Figure 1), in which daily grazing time was reduced in infested animals, but intake per bite increased to compensate and the net result was no reduction in forage intake – in fact, a slight increase (Dougherty et al, 1993a).

Irrespective of any direct effects on cattle, two species of nuisance flies found in the UK are important because of their role as vectors of several pathogens. M autumnalis is a vector of the bacterium Moraxella bovis and the parasitic nematodes, Parafilaria bovicola and Thelazia species; the head fly, Hydrotaea irritans, is implicated in the transmission of summer mastitis.
P bovicola, which causes cutaneous bleeding lesions, occurs in continental Europe, but, to date, has not been confirmed in the UK, while Thelazia species do occur, but are an infrequent cause of ocular disease. M bovis is the primary causative agent of infectious bovine keratoconjunctivitis (IBK), also known as bovine pinkeye, and New Forest disease, which is the most important ocular disease in cattle worldwide (Postma et al, 2008).
IBK
IBK is a highly contagious disease with a multifactorial aetiology; susceptibility to the causative bacteria and their transmission can involve several factors such as UV light and face flies. The importance of face flies was demonstrated in a study (Figure 2) in which fly counts on the heads of cattle were positively associated with the incidence of pinkeye in cows (Cheng, 1967).
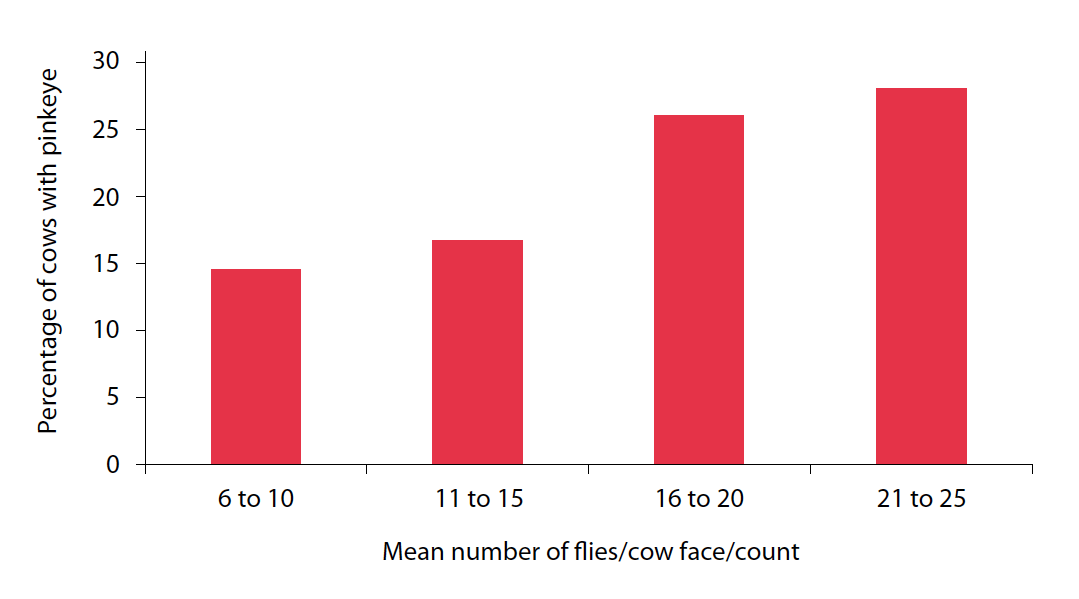
Although M bovis can survive on the exoskeleton of M autumnalis (Steve and Lilly, 1965), it is likely transmission is largely through regurgitation of crop contents containing M bovis while the insect is feeding on the conjunctiva, bacterial colonisation being aided by the abrasive mouthparts of the flies (Glass and Gerhardt, 1984).
Summer mastitis
Summer mastitis is a disease seen mostly in dry cows and heifer, commonly as a result of infection with Arcanobacterium (formerly Actinomyces and Corynebacterium) pyogenes and Peptococcus indolicus (Hillerton and Bramley, 1989).
The disease occurs in the summer months – typically from July to September on low-lying, sheltered pastures – and is strongly associated with the sheep head fly, Hydrotaea irritans. Head flies can carry the causative bacteria for several days (Bramley et al, 1985; Madsen et al, 1991), feed around the belly and udder, and are active during the months when the disease occurs.
Definitive proof this fly is the main vector of summer mastitis was difficult to confirm (Hillerton et al, 1990; Madsen et al, 1991), but researchers were able to reproduce the disease by allowing head flies, carrying bacteria from clinical cases of summer mastitis, to feed on heifers in which some of the teat canals had been traumatised (Chirico et al, 1997).
Biting flies
As the name suggests, biting flies feed on cattle primarily through blood sucking and can cause direct effects on host health, behaviour and performance. Two examples relatively common in the UK are the stable fly and the horn fly, although both have a bigger impact in the Americas, which much of the scientific literature emanates from.
Evidence exists of behavioural responses in cattle to stable flies, some of which may be detrimental to production, although the evidence is equivocal (Dougherty et al, 1993b; Mullens et al, 2006); nevertheless, both reduced growth rates and milk yield have been reported in infested cattle (Bruce and Decker, 1958; Cheng, 1958; Taylor et al, 2012a). Research in the US has also shown horn flies are integral in the pathogenesis of Staphylococcus aureus mastitis in dairy heifers (Ryman et al, 2013).
Changes in behaviour have been reported in cattle exposed to high numbers of horn fly (Harvey and Launchbaugh, 1982), although, in common with observations from other studies with flies in grazing animals, it appears, despite the obvious irritation of the animals in response to fly attack, cattle may be able to compensate, at least in part (Boland et al, 2008), so behavioural changes cannot consistently account for reported production losses to horn flies, which are inconsistent (Oyarzun et al, 2008).
Fly control in pastured dairy cattle
The principles that underpin the long-term control of parasite infections are embraced in integrated parasite/pest management (IPM) programmes (Dent, 2000).
One of the primary aims of IPM is to reduce dependence on insecticides and acaricides, in part to reduce the risk of resistance developing to the available chemicals, but also that the target parasites have to adapt to multiple avenues of control and hence cannot adapt so readily.
Insecticides
Characteristics of ectoparasiticides licensed for use in cattle in the UK are summarised in Table 2 (NOAH, 2015). It is apparent from this list the options for ectoparasite control in cattle at grass are limited as, essentially, all are from the same family, the pyrethroids, although several macrocyclic lactones have claims for horn fly control.
| Table 1. Summary information of ectoparasiticides that can be used in cattle at grass licensed in the UK | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chemical | Formulation | Face fly | Head fly | Stable fly | Horn fly | Midges | Ticks | Milk withhold |
| Alphacypermethrin | Pour-on | X | X | X | X | Zero | ||
| Cypermethrin | Ear tags | X | X | X | X | Zero | ||
| Deltamethrin | Pour-on | X | X | X | Zero | |||
| Spot-on | X | X | X | X | Zero | |||
| Permethrin | Pour-on | (X) | (X) | X | X | (X) | Zero | |
| Doramectin | Pour-on | X | 60 days* | |||||
| Eprinomectin | Pour-on | X | Zero | |||||
| Injection | X | Zero | ||||||
| Ivermectin | Pour-on | X | 60 days* | |||||
| Moxidectin | Pour-on | X | 6 days | |||||
| * Not to be used in lactating dairy cows, only in dry cows or heifers at least 60 days prior to calving. | ||||||||
The licensed claims vary somewhat according to individual product marketing authorisations and the same active ingredients may be effective against a wider range of arthropod species than those listed, although possibly in different formulations, or at different concentrations and dosages.
Decision making on fly control in dairy cattle at grass
Because of the limited resources available, it is important insecticides are not used indiscriminately for the control of pest flies in cattle. At the farm level, this can be considered at two levels.
Do direct effects of fly species present justify treatment?
The scientific evidence for direct effects of both nuisance and biting flies on weight gains in young cattle and milk yield in cows is equivocal. This may reflect conditions in the studies and it is likely the magnitude of the fly populations cattle are exposed to plays an important role.
In North America, an economic threshold for treatment of 200 flies/animal has been proposed in horn fly infestations, but even the use of this parameter does not guarantee a response to treatment (Oyarzun et al, 2008). Nevertheless, the use of thresholds is justified in providing an objective measure for determining if treatment is warranted. Given effects on performance are part of the rationale for treatment, it would also make sense to monitor growth rates in heifers and milk yield in cows to determine if these are on target.
It may be in the UK, despite the obvious annoyance and behavioural changes sometimes observed in cattle at grass, the lack of consistent evidence for direct economic impact in cattle from flies suggests routine treatment with insecticides should not be required.
Are vector-borne diseases present at levels to justify control?
Summer mastitis is a serious disease that carries a very poor prognosis, even if non-fatal, as affected animals will almost inevitably be culled because of the loss of a functional quarter, as the disease can affect replacement heifers, as well as dry cows. This could represent a serious loss of genetic potential in a dairy herd.
Control of head flies during months when they are active – typically July to September – through the use of topical pyrethroids or two insecticidal ear tags can reduce fly populations locally and reduce the risk of summer mastitis. Measures to ensure the integrity of the teat canals remains intact is also important, as head flies cannot transmit bacteria effectively in the absence of breaches in the skin barrier.
Pinkeye is clearly a very painful condition (Dewell et al, 2014) that has pronounced effects on the demeanour
and performance of affected cattle (Thrift and Overfield, 1974; Figure 3). It is, therefore, entirely justifiable to aim at high levels of
control through the avoidance and treatment of face flies.

These flies are more commonly found in low-lying, sheltered pastures, so keeping stock away from such ground, if feasible, should reduce exposure to face flies and, hence, the risk of IBK. If this is not possible then treatment with an insecticidal ear tag in each ear or application of a topical pyrethroid should effect a reduction in fly populations and reduce the risk of IBK.
Complementary measures to control pest fly populations
The treatments listed so far are effective against adult flies; however, fly larvae are also potential targets for control. Horn and face flies lay their eggs in fresh dung, where they hatch and develop through larval stages prior to pupation and the emergence of adults.
In some countries, feed-through larvicidal products toxic to fly larvae are available (Miller et al, 1978), although their use is generally restricted to intensive operations such as feedlots, rather than in grazing animals. Pyrethroids and macrocyclic lactones can reduce the numbers of fly larvae in fresh faeces (Floate et al, 2001; Mann et al, 2015); such activity is incidental to the main indications of products containing these actives and needs to be considered in the context of unintended effects on non-pest, invertebrate dung fauna (Floate et al, 2005).
Larval control in head and stable flies, which breed in a variety of habitats, including manures and rotting vegetation, is much more difficult, but in the US it has been shown areas where round-bale feeders are located provide good habitats for larval development of stable flies and attention to these sites can reduce breeding success (Broce et al, 2005; Taylor et al, 2012b).
Fly control in dairy parlours is important to avoid annoyance to the cows, the milking personnel and in the interests of dairy hygiene. A variety of spray-on insecticidal products for the treatment of premises can be used in dairies, but non-chemical methods of control are also available and may be preferable. Fly screens at the entrance of parlours, high-speed fans, fly zappers, fly papers and traps are just some examples of effective methods of fly control in buildings.
Current and potential developments in fly control
In terms of insecticides, it is somewhat ironic the burgeoning market in pet parasiticides has resulted in a plethora of new actives and combinatorial chemistry, but this has probably been at the expense of research into new ectoparasiticides for farm animals. The reasons include profit potential and regulatory hurdles for pharmaceuticals in food animals, which are generally not required in pets, such as tissue residue studies to calculate meat and milk withdrawal periods, and studies to support environmental impact assessments.
Interest has been renewed in the use of essential oils derived from bioactive plants in the veterinary control of ectoparasites (Ellse and Wall, 2014) and some blends have been shown to have repellent properties (Lachance and Grange, 2014). It is not clear if such products have to go through the full gamut of the registration procedure for veterinary products.
Other avenues for fly control that have been explored – mainly in other parts of the world, some of which have been implemented in both agriculture and horticulture – include:
- selecting animals with phenotypes/breeds less affected by flies, such as those with non-pigmented skin around the eyes and face
- breeding animals that appear to be innately resilient to fly attack
- use of non-chemical, automated systems for fly control at pasture (Denning et al, 2014)
- biological control using:
- fly parasites
- parasitoids
- entomophagous nematodes or fungi
- vaccines:
- against biting flies
- against the pathogens they transmit:
- louping ill (available in the UK)
- M bovis vaccine is available in the US
Conclusion
No quick and easy fixes exist for the control of ectoparasites of cattle at pasture and a very limited range of insecticides and acaricides are available, so treatments should be considered within the ethos of responsible use and any complementary actions that can help mitigate the impact of parasites and pest should be incorporated, where feasible.
