19 Oct 2015
High value in controlling BRD
Tim Potter explores the causes of an illness that costs the global cattle industry millions of pounds every year – and why treatment should aim to reduce the risk factors.

Figure 1. Environmental factors such as overcrowding are known to contribute to animals succumbing to disease.
Bovine respiratory disease (BRD) is the most common and costly disease affecting cattle across the world, with the estimated annual impact exceeding US$3 billion (£1.97 billion)1.
There are many bovine respiratory pathogen vaccines, new antimicrobial drugs and a greater understanding of the pathogenesis of BRD.
Transmission of infectious agents, and proliferation of endogenous microbes, often occur when cattle are subjected to stresses such as weaning, transport, overcrowding (Figure 1) or commingling with animals from other sources.

This results in damage to the respiratory tract, with subsequent upper or lower respiratory disease.
Causes
BRD is a complex, multifactorial disease involving an interaction between several factors:
Host factors
These are the characteristics that make an animal more prone to disease. They include age, immune status, nutritional status, genetics and prior exposure to the pathogens.
Environmental factors
The role of the environment in the BRD complex is well documented with factors such as transport, mixing animals from different sources, extremes of temperature, overcrowding and poor ventilation all contributing to animals succumbing to disease.
Infectious agents
Many pathogens are associated with the development of BRD:
- viruses, including bovine herpesvirus, bovine parainfluenza virus, bovine respiratory syncytial virus, bovine viral diarrhoea virus (BVDV) and bovine coronavirus (BCV)
- bacteria, including Mannheimia haemolytica, Pasteurella multocida, Histophilus somni and Mycoplasma species
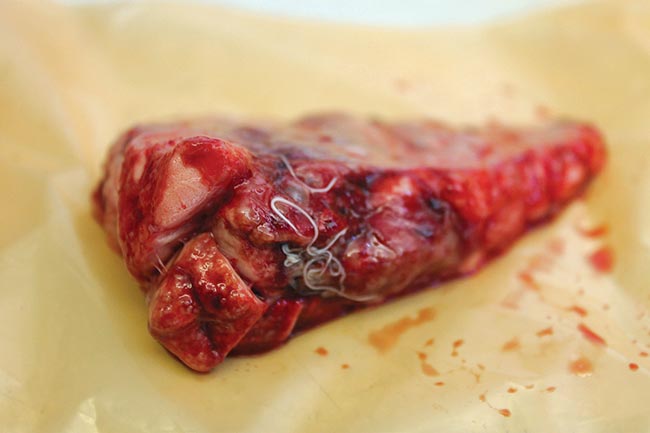
- parasites, including lungworm (Figure 2)
BCV
This is a pneumoenteric virus, which infects the upper and lower respiratory tract and intestine. It is shed in faeces and nasal secretions.
There are three distinct clinical syndromes in cattle associated with BCV: calf diarrhoea, winter dysentery (WD) with hemorrhagic diarrhoea in adults, and respiratory infections in cattle.
All BCV isolates examined to date, regardless of clinical origin, belong to a single serotype based on virus cross-neutralisation tests4.
The impact of BCV as part of the BRD complex has been increasingly acknowledged since 19954, with identification of the virus in association with disease outbreaks and reduced growth performance.
BCV has been implicated as a cause of mild respiratory disease in younger calves, with clinical signs including coughing, fever, rhinitis and inappetence. This can occasionally be seen with concurrent diarrhoea.
In older animals where the infection is frequently associated with external stressors, such as transport or mixing of animals from different sources, the signs can be more severe with fever, dyspnoea, and inflammatory and necrotising lung lesions leading to bronchopneumonia, weight loss and, in severe cases, death.
BCV infection can be diagnosed by PCR on nasal swabs. However, the acute transient nature of BCV infections, and the potential for secondary infections, necessitates sample collection at disease onset or shortly thereafter.
Samples should always be collected from animals in the acute stage of the disease.
BVDV and respiratory disease
Although it is difficult to confirm by direct experimental evidence, several lines of research indicate BVDV may be a pivotal component in BRD5 – either as a direct cause or in association with other respiratory pathogens.
While in some situations acute BVDV infections may directly result in respiratory disease, the biggest impact of BVDV infection in terms of respiratory disease is as a potentiator for secondary infections.
It is suggested BVDV potentiation of BRD occurs by two mechanisms – immunosuppression and synergism.
The immunosuppression associated with BVDV infection results from a combination of lymphoid cell death and reduced function in remaining lymphoid cells. It leaves the affected animal at increased risk of secondary infection by another pathogen component of the BRD complex.
Synergistic effects have been reported between BVDV and several viral and bacterial pathogens associated with BRD. Such synergism may occur by several different routes and depends on the pathogen in question, as well as the target host tissue.
Possible results of synergistic reactions include increased dissemination of the pathogen within target tissues (as is seen in co-infection with BVDV and infectious bovine rhinotracheitis) and a reduction in immune cell function.
Treatment
Published literature contains a large body of knowledge about the benefits of antimicrobial therapy in the treatment of BRD. Therefore, the question is probably not whether to treat with an antimicrobial, but rather which antimicrobial works best.
There is no simple answer to the latter question. Within the literature there remains a lack of studies directly comparing one product with another in naturally occurring disease situations.
The meta-analysis by O’Connor et al6 used a mixed treatment comparison meta-analysis to compare the efficacy of antimicrobial treatments of BRD.
The publication offered comparisons of 60 trials of active drug to negative controls and 33 comparisons of active-to-active (one antimicrobial to another) controls.
Through the meta-analysis method, they could rank antimicrobial treatments of BRD by efficacy. Using their rankings, with the published data available in the literature, tulathromycin ranks as the most efficacious treatment of BRD.
The information from the literature, while useful, must be used with information from the clinical picture when deciding the appropriate therapy.
There are several considerations when prescribing antimicrobial therapy, all of which need to be taken into account when managing treatment recommendations for an outbreak of BRD:
- selecting an antimicrobial agent with an appropriate spectrum of activity
- using a dosage protocol that attains and maintains an effective therapeutic concentration at the site of infection
- treating for an appropriate duration
These will have to also be balanced with economic and management constraints, as well as best practice in terms of prudent use of antimicrobials.
Little evidence exists of changing patterns of antimicrobial resistance for the major bacterial pathogens associated with BRD, but this should not drive complacency.
Development of widespread resistance to the primary therapeutic antimicrobial agents, by BRD pathogens, would be economically devastating to the cattle industry.
With the sector unlikely to see novel antimicrobial classes for BRD therapy in the near future, it is important we preserve the efficacy of products available to us.
Ancillary therapy
When considering the therapeutic management of BRD it is important to remember the role inflammatory cells, endotoxins released by Gram-negative bacteria, and cytokines have in the pathophysiology of the disease.
It is often these components of the complex that cause the common clinical signs (fever, depression, anorexia and abnormal respiratory function).
NSAIDs are often employed in BRD management to reduce the severity of clinical symptoms, increase appetite and decrease the lung damage associated with inflammation.
The use of NSAIDs as an ancillary treatment results in a more rapid decrease in rectal temperature – and data suggests NSAIDs may decrease lung lesions at slaughter8.
The complex nature of BRD, and the lack of large scale clinical trials, mean there is insufficient data to conclude a potential positive effect of NSAID use on long-term productivity and, consequently, on the positive cost benefits of such treatments. However, it is my opinion the initial clinical improvement seen with NSAIDs justifies their use from an animal welfare perspective alone.
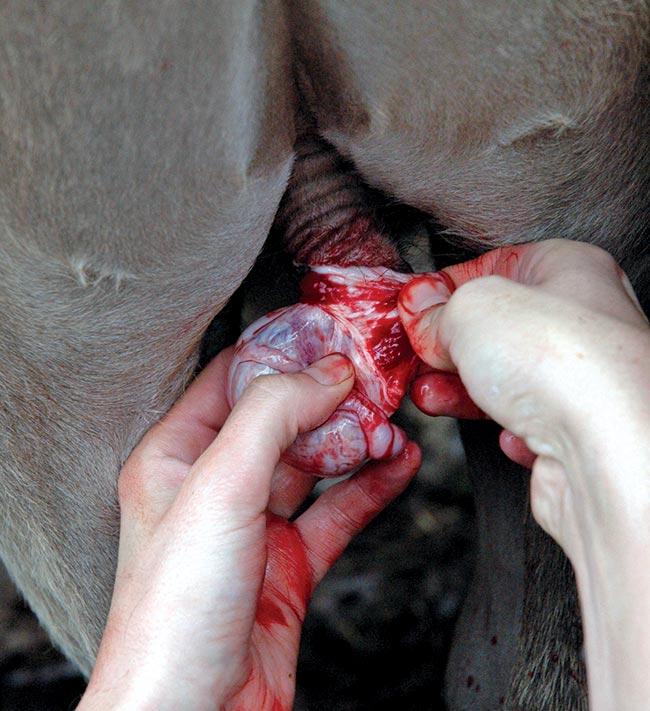
Given the multifactorial nature of BRD, the use of ancillary treatments to reduce risk factors, such as stress associated with routine management procedures (for example, castration [Figure 3] and dehorning), should also be considered.
Recent work in the US demonstrated bull calves receiving meloxicam prior to castration were almost 50% less likely to develop BRD compared with placebo-treated controls.
Conclusion
It is evident BRD is, and will continue to be, an extremely costly condition to the beef and dairy industries. Control measures should be aimed at reducing risk and must take into account the multifactorial nature of this disease complex to be effective.
