3 Sept 2018
Jacqueline Matthews describes the factors that cause this disease, clinical signs, resistance to medicines, and treatment and control methods.
Worldwide, fasciolosis is a clinically and economically important disease. It has been estimated to cost the global livestock industry in the region of €2.5 billion (£2.25 billion) each year and, in the EU, annual losses have been estimated at around €1.1 billion to €2 billion (£0.99 billion to £1.8 billion)1.
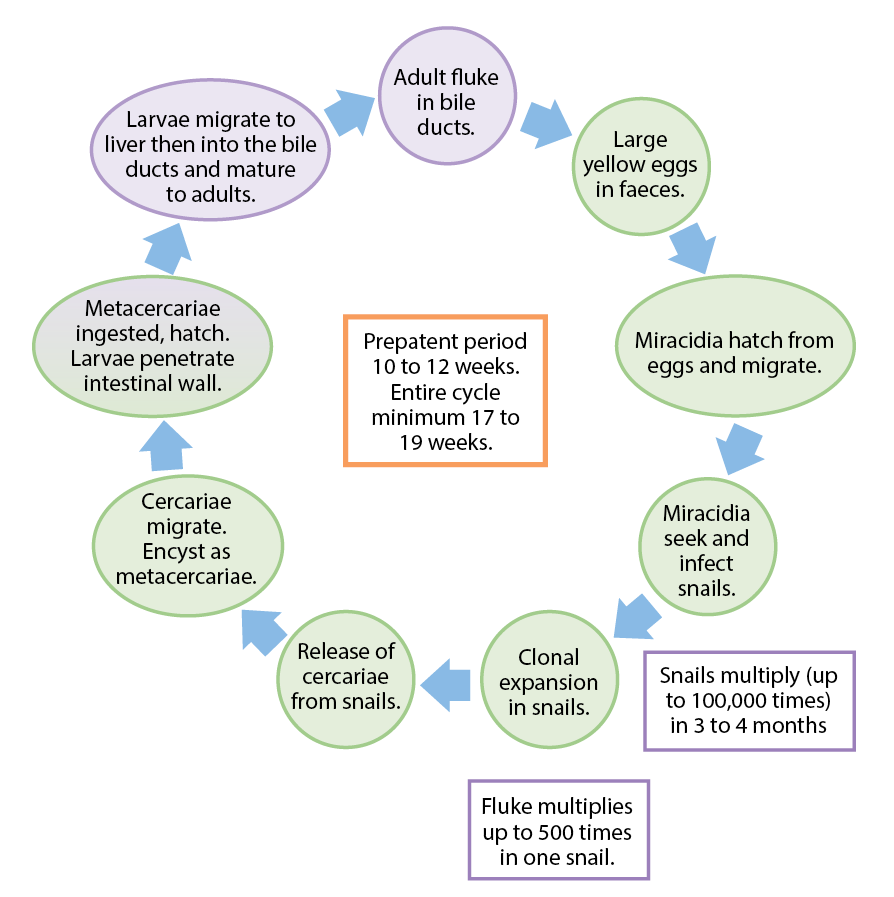
The causative agent in many regions is Fasciola hepatica. This parasite has a wide host range – including humans. It is a significant pathogen of cattle and sheep, and has an indirect life cycle (Figure 1), which includes an intermediate mud snail host (in the UK; Galba truncatula).
The environmental requirements of the pre-parasitic stages of liver fluke and its mud snail intermediate host determine the epidemiology and distribution of the parasite. F hepatica is prevalent in regions that have a wet climate and where soil is mildly acidic. In the UK, the prevalence has increased over the past 10 to 15 years. Coincident with this, an expansion has occurred in its geographic spread, from wetter western regions to traditionally drier eastern regions. This is thought to be associated with the following factors:
Environmental conditions (average daily temperature of more than 10°C and high moisture) are key for the development of F hepatica and the mud snail, and for this reason fasciolosis is seasonal, with disease outbreaks observed predominantly in autumn and winter. The incidence of disease is highest when rainfall is high in May, June and July.
Transmission to ruminants is linked to snail infection patterns (Panel 1). In some regions, wildlife hosts may also play a role in spreading liver fluke.
Summer infections in snails.
Winter infections in snails
The disease takes different forms in cattle and sheep (Panel 2). In the former, it invariably presents as a chronic syndrome, leading to production loss, low plasma protein and anaemia. In sheep, fasciolosis can develop as subacute, acute and chronic disease – depending on the fluke burden and the rate of uptake of metacercariae.
Black disease is an acute fatal disease of ruminants often associated with liver damage caused by migrating F hepatica. This damage provides an environment for germination of Clostridium novyi type B bacterial spores.
Acute fasciolosis (August to December)
This can present as sudden death due to extensive haemorrhage and liver damage. In severe outbreaks, up to 10 per cent of sheep may die. No other signs may exist. Other animals may be lethargic, dyspnoeic, graze less and reluctant to move due to abdominal pain. Fluke challenge in excess of 1,000 fluke larvae/sheep.
Subacute fasciolosis (October to January)
Rapid weight loss, anaemia, submandibular oedema, ascites and poor fleece quality. Some animals present with depression, anorexia and weakness, and some are unable to stand. Fluke challenge 500 to 1,000 flukes (various stages).
Chronic fasciolosis (January to April)
Progressive weight loss, poor condition score, poor fleece quality. Some present with submandibular oedema, diarrhoea and ascites. Some animals die in an emaciated state, especially when other conditions exist (late pregnancy/early lactation, poor nutrition, other helminthoses, infections such as Johne’s disease or foot rot). Fluke challenge 200+ adult worms.
Cattle
In dairy cows, severe chronic infection results in reduced milk yield/fertility, weight loss and sometimes diarrhoea. Signs are similar in beef cows and can be severe in spring calvers when infection worsens the physiological demands of late pregnancy/lactation. Calves may fail to thrive with perinatal losses. Affected cows can develop metabolic disease at calving or be more susceptible to other infections. Severely affected cows develop anaemia. Peripheral oedema is uncommon. Bulls and fattening cattle show similar signs. In 12-month-old to 18-month-old cattle, increases in liver condemnations are reported. In low level infections, condemnations are reported in the absence of overt clinical signs.
Treatment of acute fluke infections requires an anthelmintic active against young juvenile stages of F hepatica (Table 1). Only triclabendazole has licensed activity to kill early stages in drug-sensitive populations. Closantel, nitroxynil, oxyclozanide and albendazole are not effective against early juveniles and should only be used to treat chronic fasciolosis.
| Table 1. Efficacy of anthelmintics used in UK sheep against anthelmintic-susceptible liver fluke populations (adapted from Sustainable Control of Parasites in Sheep2) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anthelmintic | Stage of infection (weeks)* | |||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Albendazole | 50% – 70% | 80% – 99% | ||||||||||||
| Oxyclozanide | 50% – 70% | 80% – 99% | ||||||||||||
| Nitroxynil | 50% – 90% | 91% – 99% | ||||||||||||
| Closantel | 50% – 90% | 91% – 99% | ||||||||||||
| Triclabendazole | 90% – 99% | 90% – 99.9% | ||||||||||||
| * Note, for some combination products (not shown here), the licence for activity against liver fluke juveniles varies. For specific information for each combination/brand, always check the SPC. | ||||||||||||||
Because of the extensive use of triclabendazole for several decades, resistance to this compound is present in many regions in the UK. For this reason, products containing triclabendazole should be reserved for cases when acute or subacute disease is identified as a threat or in an acute/subacute disease outbreak.
In the latter case, all sheep should be treated, then moved to lower-risk pasture if available. If sheep cannot be moved, they should be administered with triclabendazole three weeks after the first treatment (no flukicides have persistent anthelmintic activity) to kill further incoming infection. However, this provides a selection pressure for resistance.
Animals can still die after treatment because their livers are so badly damaged by the time disease has been detected.
In subacute disease outbreaks, sheep should be administered with an anthelmintic with licensed activity against mature and immature fluke (Table 1). Again, treated sheep should be moved to lower-risk pastures. If the group cannot be relocated, a second treatment should be administered (three to eight weeks later, depending on the product used and its spectrum of activity).
In chronic fasciolosis in sheep (Table 1) and cattle (Table 2), administer an anthelmintic that is active primarily against adult liver fluke. As in the other situations, if possible, move the group to lower-risk pasture to avoid reinfection.
| Table 2. Licensed effect of anthelmintics used in UK cattle against drug-susceptible liver fluke | ||
|---|---|---|
| Active ingredient | Stage of fluke killed | Formulation |
| Triclabendazole | 2 weeks + | Oral |
| Triclabendazole plus moxidectin* | 6 weeks + | Pour-on |
| Closantel plus ivermectin | 7 weeks + | Injectable, pour-on |
| Nitroxynil | 8 weeks + | Injectable |
| Clorsulon plus ivermectin | Adults | Injectable |
| Albendazole | Adults | Oral |
| Oxyclozanide** | Adults | Oral |
| * Note, for some combination products or routes of administration, the licence for activity against liver fluke juveniles varies. For specific information for each combination/brand, always check the SPC. | ||
| **Oxyclozanide can be used to treat rumen fluke, when prescribed by a veterinary surgeon. It is only licensed for treatment of liver fluke. | ||
Few flukicides are licensed for use in milking cows in the UK. Those licensed are albendazole and oxyclozanide, which have a 60-hour and a 72-hour milk withhold respectively. Some triclabendazole-containing products can be used in the dry period. These products have between 44-day and 50-day milk withdrawal periods. Keep in mind specific details for each formulation can change over time and varies with the brand, so always check the summary of product characteristics (SPC) for each product prescribed and used.
Cattle and sheep can become quickly reinfected after treatment when grazed on contaminated pasture as none of the flukicides have any persistent effect after administration. Note also fluke-induced liver damage can affect the pharmacokinetics of some compounds (most notably triclabendazole), so where liver damage is severe, this can impact the effectiveness of the flukicide administered in the absence of flukicide resistance.
Changes in our climate, and the emergence and spread of triclabendazole resistance, are key challenges for the effective control of F hepatica in the UK. In designing a sustainable fluke control plan, the following aspects must always be considered:
Diagnostic tests should be exploited to detect infection or diagnose disease, and to inform the need to treat individuals and to test efficacy.
Available diagnostic options include:
Fluke egg shedding levels can fluctuate daily and the eggs can be difficult to detect in low level infections because of the relatively poor sensitivity of the test. Indeed, FEC test sensitivity varies depending on the methodology used to count the number of eggs. The volume of faeces examined, in particular, affects sensitivity and this can be improved by using higher volumes of faeces or by reading multiple samples per animal. Because of these issues, it is recommended to test as many animals as possible (at least 10) when investigating for liver fluke infection.
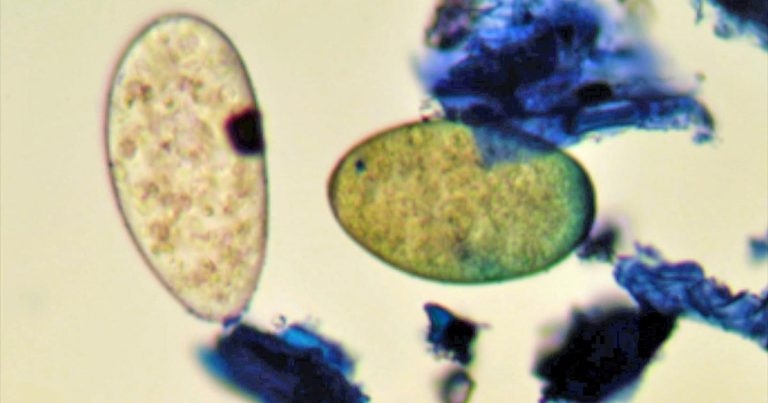
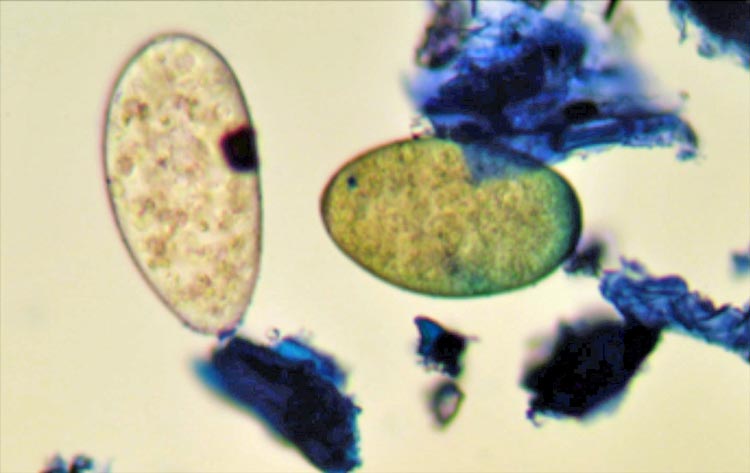
One advantage of the FEC test is it allows discrimination of liver fluke from rumen fluke eggs (Figure 2), with the former yellow and the latter colourless.
Sedimentation FEC tests are useful when infections are likely to be chronic. Diagnostic laboratories often report a positive or negative test result depending on whether fluke eggs are observed in samples, but eggs need to be counted when undertaking an efficacy test.
Published information is lacking for cattle relating to how soon the test can detect liver fluke post-infection. Further studies are required for this test’s practical application. Work, so far, suggests the F hepatica coproantigen test does not cross-react with rumen fluke antigens in cattle or sheep. The test is also useful for assessing anthelmintic efficacy when resistance is suspected.
Where fasciolosis has been identified, flukicides need to be used judiciously. Anthelmintics should be selected based on:
Triclabendazole should only be prescribed when it is deemed essential (for the treatment of sheep in acute/subacute fasciolosis outbreaks in late summer and autumn). Other types of flukicide should be selected for use in control plans to target adult worms to reduce the impact of parasites in individuals and reduce fluke egg shedding into the environment. Care should be taken when considering the use of combination anthelmintics; the nematocidal activity of these may be unnecessary at the time of year when flukicides are required. In these cases, using these products will only provide unwarranted selection pressure for anthelmintic resistance in nematodes.
As aforementioned, abattoir or postmortem data provide valuable information to inform the need to institute specific control/treatment plans. Abattoir data can be supported by FEC or coproantigen testing cattle or sheep or, in dairy cows, bulk milk tank antibody testing. Using these tests over time enables the success – or otherwise – of control plans to be assessed and informs the need for further treatments.
A key component of control is the application of land management measures to reduce snail-friendly environments on the farm. This can be achieved by fencing off muddy wet areas from grazing access. Likewise, improving drainage will help dry the environment to limit snail populations, as well as reduce development and survival of pre-parasitic stages of F hepatica. However, this can be in conflict with environmental schemes designed to protect wetland; therefore, a compromise needs to be reached.
Liver fluke control is increasingly becoming a challenge in the UK and control plans need to be designed to ensure the anthelmintics selected are, and remain, effective.
To address flukicide resistance, industry bodies promote the rotation of anthelmintic use in fluke control plans where these medicines are used strategically. Where possible, the use of triclabendazole should be avoided and alternative compounds used where it is not essential to target high numbers of Fasciola juveniles. In these cases, plans need to be adapted to account for the spectrum of activity of each compound. For example, to ensure activity against mature parasites if using closantel or nitroxynil at housing, delay treatment for three weeks or, in the case of oxyclozanide or albendazole, six weeks after housing. In a similar vein, do not use triclabendazole when targeting infections that comprise adult infections – for example, if applying treatments in spring.
Triclabendazole resistance is a major threat to the control of acute/subacute fasciolosis in sheep. Unfortunately, no alternative anti-larval products are in the development pipeline and commercially available fluke vaccines are a long way off. Moreover, field-based methods for the detection of flukicide resistance are not that well validated, so confirmation of resistance can be difficult.
Resistance is often first detected as a failure of triclabendazole to kill immature worms, which develop after treatment to produce eggs in dung earlier than would be anticipated in anthelmintic-sensitive populations. If such populations continue to be exposed to the same anthelmintic, resistance worsens and adult flukes survive treatment, with low FEC reductions observed after treatment. Methods to test conclusively for flukicide resistance are still in development; Table 3 summarises the state of play.
| Table 3. Options for detecting flukicide resistance in cattle and sheep | |||
|---|---|---|---|
| Test name | Methodology for individual samples | Methodology for pooled samples | Comments |
| Sedimentation faecal egg count reduction test | Collect 10 to 15 individual dung samples from identified animals before treatment. Administer flukicide, ensuring the full dose is swallowed. Collect post-treatment samples three weeks later. Collect individual samples from the same animals. Efficacy is reported when egg numbers fall by more than 90% to 95% after treatment. | An alternative is to use a pool of 10 × 5g individual dung samples and count all the eggs in the well-mixed 50g pooled sample. Sample and pool material from the same animals pre-treatment and post-treatment using the time interval indicated in the individual tested samples. | This test is only useful in patent infections when eggs can be detected in dung. |
| Coproantigen reduction test | Collect 10 to 15 individual samples before treating with flukicide. Ensure the full dose is swallowed. At two weeks post-treatment, collect individual samples from the same animals. This test can be used to assess triclabendazole efficacy when the fluke burden comprises late immature/adult stages. The threshold for efficacy is reported as a mean percentage positivity reduction of at least 90% post-treatment. For other flukicides the test can be adapted when burdens comprise adults. The reduction in positivity is interpreted alongside the expected efficacy of the compound against adult fluke, as reported in the SPC. | Not recommended | |
| As yet, no research guidelines have been agreed for the detection of flukicide resistance in sheep or cattle. | |||
When triclabendazole resistance is suspected or proven, an alternative class of anthelmintic should be used – keeping in mind it will have a lower spectrum of activity against liver fluke developmental stages than triclabendazole.
For animals coming on to a farm from an area where fluke is present, flukicide administration should be considered. In these cases, products that do not contain triclabendazole should be used, keeping in mind these products will not kill all the immature stages, so a second treatment four to six weeks later will be required.
Surveys have concluded farmers who are better informed, and have increased awareness of anthelmintic resistance and worm control practices, are more likely to have a positive approach to implementing best practice advice and deploy diagnostic tests on their farm4. The effective transfer of the up-to-date information by vets is a critical link in disseminating knowledge and promoting sustainable worm control measures.