29 Apr 2019
Liver fluke treatment in cattle and sheep
Sarah Hewitt discusses the life cycle of this parasitic disease, methods of detecting infection, treatment options and measures for its control.

Figure 2. Mud snails tend to be found on wet, boggy ground.
Liver fluke is a zoonotic infection that can significantly affect performance of cattle and sheep. Considering the life cycle of the intermediate host, the mud snail is vital in designing and implementing effective control protocols. Fluke infection levels are heavily influenced by climate and environment – so, to be most effective, control protocols should be farm, climate and environment-specific, and may, therefore, vary year to year and farm to farm.
Strategic use of flukicides and management options can be looked at – and with concerns regarding resistance to flukicides increasing, alternative methods of fluke control should be considered. In addition, withdrawal periods of flukicides need to be taken into account, particularly in lactating dairy cows.
Various diagnostics can be used to identify fluke infection and monitor the effect of any control plans put in place. These can be carried out routinely – allowing continuous monitoring of fluke infection on farm and adjustment of control measures as necessary.
Liver fluke (Fasciola hepatica) infections in cattle and sheep can have a major effect on production, and understanding the life cycle and distribution of the parasite is crucial in designing and implementing effective control plans.
Liver fluke is a trematode that parasitises molluscs (as intermediate hosts) and vertebrates (as definitive hosts). Cattle and sheep are the main definitive hosts of liver fluke – although deer, rabbits and humans can also act as definitive hosts – while the intermediate host is the mud snail (Galba truncatula). This means the prevalence of infection increases where cattle and sheep encounter infected mud snails – that is, on wet pasture – and tends to vary with changing environments and climates.
As their name suggests, liver fluke damage the liver. This results in a variety of clinical signs, including reduced growth rates and carcase quality, weight loss, reduced milk yield and quality, reduced fertility and death (Mazeri et al, 2017; Howell et al, 2015; Beesley et al, 2017; Charlier et al, 2014; Sargison and Scott, 2011). Production losses are further increased by liver condemnations at slaughter.
Fluke infection can also lead to immune modulation, making cattle more prone to other diseases, such as bTB, and affecting disease diagnosis and, possibly, vaccine efficacy (Charlier et al, 2014; Flynn et al, 2009).
Figures around the true financial cost of fluke infection are hard to estimate because of these varied effects on production. Costs to the UK cattle industry annually have been estimated at between £40.4 million (Control of Worms Sustainably, 2018) and £23 million (Scott, 2016), while, at the individual animal level, estimates are £25 to £30 per sheep and £200 per dairy/beef cow (Skuce and Zadoks, 2017). In addition to the economic costs, a welfare cost to liver fluke infections clearly exists.
Life cycle
The mud snail intermediate host is crucial for the fluke to complete its life cycle, so the presence of this organism dictates treatment timing and control protocols.
A single adult fluke can shed up to 50,000 eggs per day, leading to rapid and heavy pasture contamination. These eggs hatch in a temperature-dependent way to release the first larval stage – the miracidia, which infect the mud snail by burying through the foot into the abdominal cavity.
Within the mud snail, asexual reproduction takes place – producing the second larval stage, the cercariae. As reproduction is asexual at this stage, the potential exists for rapid expansion of any drug-resistant populations. These cercariae are released from the snails after about six weeks and form cysts (metacercariae), which are then ingested by definitive hosts such as cattle, sheep, humans and many other vertebrates. When ingested, juvenile fluke excyst and migrate from the gut through the liver, causing damage as they go to the bile ducts, where they mature and lay eggs. The total prepatent period is 10 to 12 weeks (Figure 1).
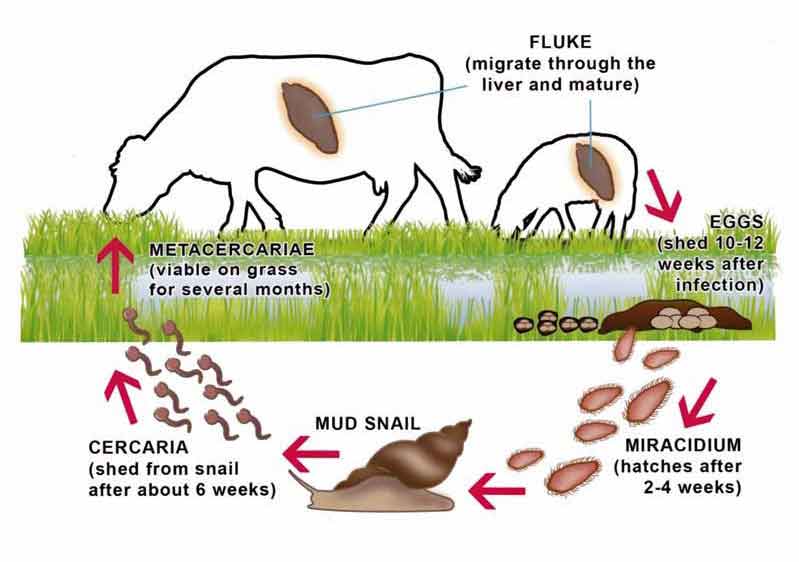
Mud snails are integral to this life cycle, so avoiding contact with them and breaking the cycle is an important aspect of control. Mud snails tend to be found on wet, boggy ground and particularly large populations can be found following wet weather (Figure 2). This leads to seasonal variation in fluke infections, with snails tending to be infected in the spring, pasture being contaminated over the summer, livestock becoming infected in the autumn and clinical signs being seen in the winter.

Fluke activity is very climate-dependent, meaning mild/wet winters may result in animals becoming infected in the spring. This influences when periods of high risk occur and, therefore, when animals should be treated. The National Animal Disease Information Service provides fluke forecasts for different regions of the UK based on the weather and previous infection patterns, and can be used to inform treatment timing decisions. However, individual farm geography and management should also be taken into account when developing control protocols, as these can vary locally (McCann et al, 2010; Knubben-Schweizer and Torgerson, 2015).
Diagnostics
Several diagnostic methods are available for fluke detection. For long-term monitoring, abattoir reports of liver condemnations can be used, or regular bulk milk sampling for fluke antibodies in dairy herds. For individual or small groups of animals, blood or faecal sampling may be carried out. Blood samples can be used to detect antibodies created against a fluke challenge, which takes around two to four weeks (Salimi-Bejestani et al, 2005). These antibodies can persist, however, with detection 18 months after infection being reported (Hutchinson and Macarthur, 2003), meaning results cannot be relied on to indicate recent or current infection. Analysing liver enzymes in blood samples may also be used to identify liver damage or assess function; however, this is not fluke-specific and how well it correlates with prognosis can be hard to ascertain (Skuce and Zadoks, 2013).
Faecal testing typically detects fluke eggs, so will only detect infection after the prepatent period (10 to 12 weeks). Faecal egg counting can also produce false-negative results due to low sensitivity (although repeat testing has been shown to improve this), and storage of eggs within the gall bladder with intermittent shedding (Charlier et al, 2014; Sargison and Scott, 2011).
The coproantigen ELISA is a newer test that detects fluke antigens in faeces, so can detect infection before the end of the prepatent period. However, field trials have sometimes struggled to replicate detection rates achieved experimentally, particularly in cattle (Skuce and Zadoks, 2013). In acute infection, where sudden death is a common presenting sign, a postmortem is often the best method of diagnosis.
Clearly, all diagnostics have their limitations, and these should be considered when investigating fluke infection patterns on farm and using results to inform management decisions. Identifying particular groups of animals with fluke infection (or stage of production at which animals are becoming infected) can be used to help inform these decisions, and to identify high-risk areas of pasture, times of year and groups of animals (Knubben-Schweizer and Torgerson, 2015). Regularly repeating diagnostics and reviewing fluke control, with respect to results and recent climate, can aid in tailoring control strategies to individual farms each year.
Treatment
Liver fluke infections may be chronic (Figure 3) or acute (Figure 4), depending on the infective dose ingested, and the type of infection determines the most appropriate treatment options.
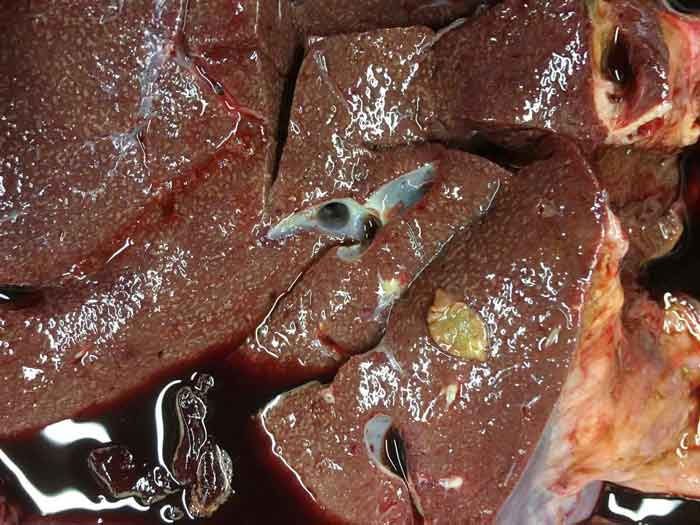
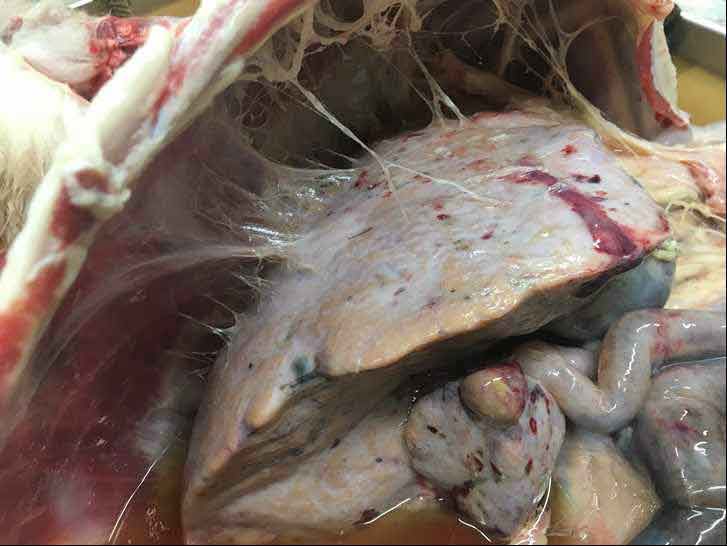
Sheep are more likely to show signs of acute infection (severe liver damage, anaemia and sudden death) after ingesting large amounts of infective cysts – often in the autumn. Deaths may occur before the immature fluke have developed into egg-laying adults (that is, during the prepatent period), meaning fluke eggs may not be detected in the faeces. Chronic fluke infections are seen in both sheep and cattle, typically in winter/early spring following prolonged intake of low numbers of infective cysts. Signs of chronic fluke infections include weight loss, bottle jaw and poor fertility.
Considering the spectrum of activity of the flukicide – that is, the stages of the fluke life cycle it targets with respect to the type of infection present – is important when selecting treatments. Other aspects to consider include recent weather conditions, time of year, land grazed, likely infection pressures and milk/meat withdrawals. It is unlikely following the same protocol on every farm, or even on the same farm in subsequent years, will be the best option. Control strategies should be tailored to individual farms and reviewed regularly. This is particularly pertinent regarding the use of triclabendazole, as cases of resistance to this flukicide have been reported in sheep and cattle (Sargison and Scott, 2011; Skuce and Zadoks, 2013).
Triclabendazole is often used as a first-line treatment and control for liver fluke, as it kills the widest range of fluke life cycle stages. However, it is not always required – for example, if cattle have been housed for a period and only adult fluke are likely to be present. In fact, it has been suggested triclabendazole shouldn’t be used in cattle at all, and should be reserved for treating sheep where acute fluke infection – and, therefore, targeting of the immature stages of the parasite – is more common (Skuce and Zadoks, 2013).
Other treatment options include sequential treatments with a drug killing adult fluke only, although this will only be effective if animals are not subsequently reinfected. In contrast to wormers, flukicides don’t have persistent activity, so cattle and sheep can get reinfected from infected pasture if returned to it following treatment. Also, in contrast to roundworms, cattle and sheep don’t generate effective immunity to fluke, so older stock that have previously been exposed to fluke cannot be presumed to be immune.
When co-grazing cattle and sheep, it is important to remember the same parasite can infect both species, and treatment/control protocols should include both cattle and sheep. Wildlife reservoirs of infection may also mean fluke populations can be maintained in the absence of livestock. The use of combination products, often combining flukicides with other wormers, should be considered carefully to ensure risks of promoting resistance to other products are minimised.
Withdrawal periods have to be considered when selecting treatment options, and this can be particularly problematic when treating dairy cows. Two active ingredients are licensed for treating dairy cows – albendazole and oxyclozanide – and both kill adult fluke (10 weeks post-infection onwards), meaning repeat treatments may be required. This may be challenging to implement as milk withdrawals will be required after each treatment. Another alternative is to treat cows at drying off with an appropriate product (Table 1).
| Table 1. Flukicides for cattle and sheep in the UK | ||||
|---|---|---|---|---|
| Active ingredient | Administration route | Age of fluke targeted | Cattle or sheep? | Use in dairy cattle |
| Triclabendazole | Oral | Two weeks onwards (some products from a few days of age) | Cattle and sheep | Some products may be used during the dry period (withdrawal periods apply) |
| Pour-on | Six to eight weeks onwards | Cattle | None | |
| Closantel | SC injection (in combination with ivermectin) | Seven to nine weeks onwards | Cattle and sheep | None |
| Pour-on (in combination with ivermectin) | Seven weeks onwards | Cattle | None | |
| Oral | Five weeks onwards (activity from three weeks with some products) | Sheep | N/A | |
| Nitroxynil | SC injection | Seven to eight weeks onwards | Cattle and sheep | None |
| Clorsulon | SC injection (in combination with ivermectin) | Ten weeks onwards | Cattle | None |
| Oxyclozanide | Oral | Ten weeks onwards | Cattle and sheep | Milk withdrawal 108 hours |
| Albendazole | Oral | Ten weeks onwards | Cattle and sheep | Milk withdrawal 60 to 72 hours |
| Adapted from Control of Worms Sustainably flukicide products for cattle and using information from the NOAH Compendium. Correct as of November 2018. For the latest information, refer to the VMD product information database | ||||
Monitoring treatment success
When monitoring treatment success, it is important to differentiate between drug resistance and treatment failure (Skuce and Zadoks, 2013). Treatment failure may be due to incorrect dosing of cattle or storage of the product, rather than resistant fluke populations. Immediate reinfection of animals may also mimic apparent treatment failure.
It is also worth considering that products licensed to target different stages of fluke often don’t report 100% efficacy at these stages (particularly the immature stages). Drug resistance can be detected using faecal egg count reduction tests (FECRTs). The risk of false-negative results with FECRTs, however, may sometimes indicate apparent treatment success in the absence of full efficacy, so results should be interpreted in light of this. FECRTs have been shown to correlate poorly with levels of fluke infection identified at postmortem examination, although the coproantigen ELISA has been suggested as an alternative test for treatment success (Skuce and Zadoks, 2013; Beesley et al, 2017). Molecular tests under development may offer more reliable identification of drug resistance in fluke populations in the future (Beesley et al, 2017; Skuce and Zadoks, 2013).
Other control measures
Breaking the life cycle of the fluke – that is, preventing, contact between cattle and snails – is key to implementing a control strategy; therefore, pasture management can play an integral part. Although climate clearly influences fluke levels temporally and geographically, management factors have been shown to be associated with fluke levels to much the same degree (Howell et al, 2015) – for example, fencing off or draining boggy pasture and avoiding livestock grazing fluke habitats. Admittedly, this is sometimes challenging; these areas can be difficult to identify, with the snails – at only 5mm to 6mm long – often hard to find.
Areas of fluke habitat may also change throughout the year or between years, and fluke may travel outside their “habitat” (Knubben-Schweizer and Torgerson, 2015). Drainage of boggy pasture to reduce snail populations may be an option; however, this sometimes conflicts with environmental conservation schemes. Rotation of grazing to limit pasture/snail contamination may be an option, and strategic dosing may be used with the aim of reducing snail and pasture infection levels – for example, treating animals in the spring before turnout to prevent snail infection (although the presence of wildlife reservoirs may compromise this approach). Quarantining bought-in animals, even if fluke are known to be present on the farm, is of importance to avoid bringing in resistant fluke populations.
Two treatments of a product targeting adult or intermediate fluke stages – used 6 to 10 weeks apart, depending on the product used – can achieve this without the need for triclabendazole. Other potential control methods include the development of a vaccine, and although progress is being made in this area, it is not an option available to us (Molina-Hernández et al, 2015; Beesley et al, 2017).
Conclusion
Fluke control is complex due to the many varied factors that can influence infection pressures and disease levels on farm (Table 2). Imperfect diagnostics mean results should be interpreted with care, and developing drug resistance and licensing restrictions mean drugs alone should not be relied on.
| Table 2. Fluke control – challenges and options | |
|---|---|
| Challenges | Options |
| Not host-specific – the potential exists for cross-infection between cattle and sheep, and from wildlife reservoirs. | Include all livestock in control protocols and consider potential impact of wildlife reservoirs. |
| Infection levels are influenced by climate and geography, so vary year to year and farm to farm. Overall levels are increasing and areas previously unaffected are now detecting fluke infections. | Although heavily influenced by climate and environmental factors, management steps can be taken: • Reduce pasture contamination – for example, treating in the spring before turnout. • Reduce snail population – for example, by drainage where appropriate. • Avoid contact between cattle and snails – fence off/avoid grazing high-risk pasture at high-risk times (generally in the autumn). • Repeat diagnostics and review treatment protocols regularly, depending on current infection patterns. |
| Habitat and snails may be difficult to identify. | Consider testing different groups of animals to identify when and where infection occurs. |
| Drug resistance (and detecting it). | • Monitor treatment success, and differentiate between treatment failure and drug resistance. • Consider the type of infection present (acute/chronic) and use an appropriate product. • Reserve triclabendazole for acute fluke infections, where possible. • Treat and quarantine brought-in stock to avoid bringing in resistant fluke populations. |
| No persistence of flukicides. | Animals should be assumed to be at risk of reinfection post-treatment. Consider housing/avoiding contact with snails post-treatment. |
| Cattle and sheep don’t appear to develop any protective immunity to fluke infection. | All ages of livestock should be assumed to be at risk of infection when developing control protocols. |
| Immune modulation – may affect animals’ responses to other diseases and testing for these diseases. | This is an area being investigated, with progress being made towards better defining the effects of the parasite on the host immune response and towards development of a vaccine. |
Pasture management can clearly play a part in fluke control, but may be challenging to implement due to farm level practicalities. Control protocols are likely to vary farm to farm and year to year, and engaging with farmers to regularly review these – and develop dynamic and holistic control strategies – is likely to be of benefit in limiting production losses.
