17 Jun 2025
Mastitis – technology use and the importance of accurate diagnosis
David Charles CertHE(Biol), BVSc, CertAVP(Sheep), PGCertVPS, MRCVS considers the financial impact of this prevalent condition, and explores how practitioners can test for it with confidence.
Image: Casa.da.Photo / Adobe Stock
Mastitis (inflammation of the mammary tissue) is a condition that any production-animal vet or farmer worldwide will be familiar with, and much has been published on the subject.
This article will provide an overview of the condition, the importance of accurate and correct diagnosis, as well as considering new technological advancements that can aid diagnosis and, therefore, advice relating to its treatment and prevention.
What should clients be targeting in terms of occurrence?
Recommended targets relating to somatic cell count (SCC), and clinical and subclinical cases include:
- Less than 20% of cows with SCC more than 200,000cells/ml.
- Clinical mastitis rate less than 35 cases per 100 cows.
- Bulk tank SCC less than 150,000cells/ml.
- Less than 5% of cows with chronic high SCC.
- Dry period cure rate more than or equal to 85%.
- Dry period (fresh calver) new infection rate less than 8% (1 in 12).
- Lactation period new infection rate less than 16% (2 in 12; Breen, 2014; GB Cattle Health and Welfare Group, 2020).
Economic impact
The economic impact of mastitis on dairy farms worldwide is vast, with losses being multifactorial.
Economic losses arise from: lost milk production, discarded milk, medication, diagnostic testing, farm worker time, culling costs, reduced fertility, and veterinary time (this is a non-exhaustive list). In the worst cases, farmers can also be financially penalised by their milk buyer.
Worldwide, mastitis is believed to cost the dairy industry £13.7 billion to £22.3 billion per year (converted from Euros; Ceva Animal Health, 2018). In the UK, the cost is estimated at £173 million per year (Hyde et al, 2020).
On a local level, clinical mastitis is estimated to cost farm businesses £200 if milk is worth 25 pence per litre (ppl) or £400 at 50ppl (Gard, 2023). With milk prices rising in recent years, so too has the price of mastitis.
One veterinary practice estimates that for a “typical” 200-cow UK herd, the average case rate of 32 cases per 100 cows per year and a bulk tank SCC of 162,000 costs can reach an average £15,594/year.
If subclinical mastitis rates increased and SCC reached 250,000, the cost would increase to £25,029. However, reducing the case rate to the top-benchmark rate of 20 cases per 100 cows per year would save more than £5,000 per annum (LLM Farm Vets, 2021).
Diagnosis
Only through accurate diagnosis can appropriate treatment be implemented. Furthermore, without an appropriate understanding of the causative pathogens – and the most likely routes of infection – effective prevention and control measures cannot be implemented.
To the author, three key stages fall under the category of “mastitis diagnosis”. Namely these are:
- Diagnosis of the condition (clinical or subclinical).
- Identification of the causative pathogens.
- Quantifying when and where the infection is occurring.
Diagnosis of the clinical condition
Diagnosis can occur in a number of ways. Firstly, these cases may be detected based on clinical changes: it is important to note that while the clinical signs of the “acute toxic mastitis” (Panel 1) might be the ones that spring most often to the vet or farmer’s mind, more often than not, early clinical changes will be detected during “foremilking” or “stripping out” in the parlour.

Mild changes detected in foremilking would include clots, mild flecks, watery milk or changes in colour and/or consistency. Changes in the milk are often the first signs of a clinical case; therefore, close attention to foremilking routine and interpretation is paramount.
However, not all farms will utilise foremilking as a routine procedure (naturally, including those utilising automatic milking systems; AMS) and so may miss the earliest clinical signs.
Latterly in the progression of the disease, changes in the udder will be detected. Where routine palpation and visual assessment of the udder is performed during each milking, this can be a useful tool, although cases will be detected much later than if foremilking was used as a first test.
Changes observed to the udder would include swelling, erythema, heat on palpation and pain, which may exhibit as kicking on palpation or attachment of the cluster. By the time udder changes are identified, considerable losses – and potentially damage – will have occurred.
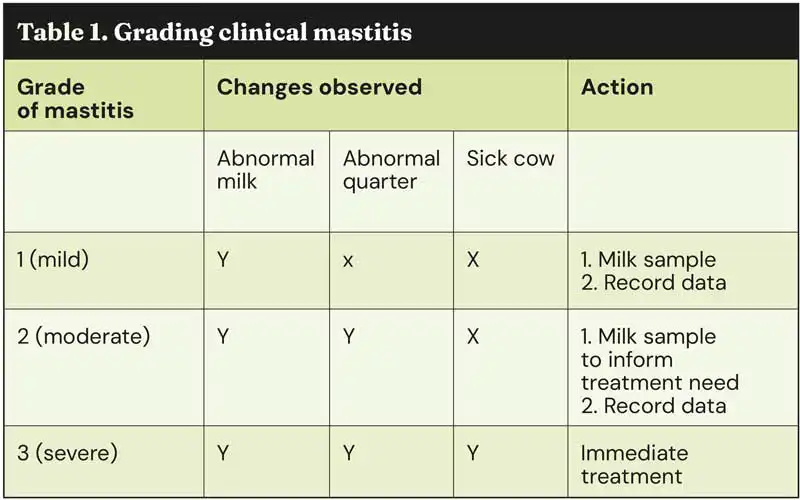
Clinical signs can be used to effectively grade mastitis into one of three categories. All vets and farmers should be familiar with the grading system, and the advice recommended for each grade (Table 1).
Diagnosing subclinical mastitis requires greater involvement from the client, but can yield great rewards in the short and long terms. To name but a few benefits, controlling subclinical cases can result in:
- Lower SCC – reducing the risk of financial penalties.
- Fewer cows developing clinical mastitis – reducing the impact of yield and improving animal health and welfare.
- Reduced antibiotic use.
Subclinical mastitis is traditionally detected through milk testing as a method to detect the impact of the inflammatory processes in the udder. The three major methods would be:
- California mastitis test (CMT) – also known as rapid mastitis test.
- SCC testing.
- Bulk tank SCC.
- Individual cow SCC.
- Electrical conductivity.
The CMT is a widely used test, first standardised in the 1960s. Due to its ease of use and low cost per test, it has been adopted worldwide. The test is performed using a four-quartered paddle, whereby milk from each quarter is expressed into the corresponding well on the paddle. Reagent is then added to each well, and the solution is agitated and evaluated against a standardised scoring system (Table 2).
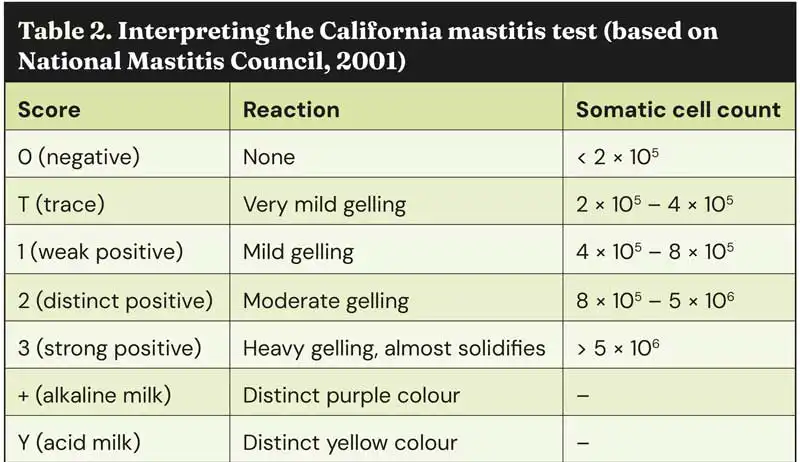
With scores corresponding to a likely SCC range, the CMT remains a useful tool for identifying infected quarters after individual cow SCC identifies cows with subclinical mastitis. This, in turn, reduces the use of unnecessary antibiotics and allows targeted treatment.
It is important to note that the CMT does not indicate the causative agents, or identify at what stage of lactation infection occurred; therefore, it cannot be used as a guide for which group of antibiotics should be given to the affected quarter.
Other limits of the test are the operator skill and the slight subjectivity of interpretation. Published works over the past 50 years have produced variable levels of specificity and sensitivity (Roberts, 2025a).
In some countries where individual cow SCC is not performed due to practical or economic reasons, each cow will undergo a CMT at a routine period for monitoring the level of subclinical mastitis in the herd. A general goal for any herd is less than 15% of cows with a quarter recording CMT 2 or above. SCC testing provides an in-depth interpretation of the quality of the milk. SCC testing can be done on bulk milk samples and on individual cow samples (ICSCC). Most often, bulk somatic cell count (BSCC) is reported by the milk buyers to the farmer on a monthly or bimonthly basis, as a measure of milk quality and udder health. BSCC should be less than 2 × 105cells/ml, with readings greater than 5 × 105cells/ml often incurring economic penalties to the farmer and indicating herd problems with subclinical or clinical mastitis.
BSCC is less reliable in smaller herds, where a smaller number of cows can have a greater impact on the BSCC reading.
Practitioners advising their clients regarding the interpretation of BSCC testing should remember that the predominant pathogen will also affect the SCC. Streptococcus agalactiae has been demonstrated to elevate BSCC more than Staphylococcus aureus. Likewise, other confounding factors will influence BSCC and ICSCC, with age and seasonality affecting SCC results.
Linear scoring (LS) is a method used to reduce the impact of fluctuations on SCC, and to provide a more accurate measure of impact – in terms of milk losses – than ICSCC alone. While the ICSCC may fluctuate test on test, the LS is less likely to vary (as a log score of the ICSCC), making it more repeatable than ICSCC alone. LS 0 correlates with ICSCC 0cells/ml – 18,999cells/ml and causes 0kgs of milk losses, while LS 5 (284,000cells/ml to 565,999cells/ml) equates to 2.04kg of milk loss/day. At the top end, LS 9 (4,524,000cells/ml to more than 9,999,999cells/ml) equates to 4.76kg of milk loss/day (Shook and Seaman, 1983).
At 46ppL (February 2025 farmgate price; AHDB, 2025), this would cost the farmer £2.12 per cow per day, or £648.18 per cow per 305 days in lost milk alone.
Electrical conductivity (EC) testing is a useful – and often underutilised – tool for detecting bovine mastitis – particularly subclinical mastitis.
Mastitis leads to changes in the milk’s electrolyte composition (owing to changes in the permeability of the mammary gland cells to electrolytes in milk), with increased Na+ and Cl– and a reduction in K+, resulting in increased EC.
Studies have shown that EC measurements can effectively identify infected quarters, with post-milking strippings often exhibiting higher conductivity than foremilk in cases of primary pathogen infections. This makes EC a promising screening method for early mastitis detection.
The efficacy of EC testing has been evaluated through various studies (Isaksson et al, 2021). A meta-analysis reported an overall sensitivity of 66% and specificity of 94% for EC in detecting mastitis, indicating its reliability as a diagnostic tool.
Additionally, research comparing EC with other indirect methods found that EC, along with sodium and chloride content, was more accurate for predicting infection status of quarters than variables such as lactose or bovine serum albumin. These findings underscore the diagnostic potential of EC testing (Fernando et al, 1982).
In practical applications, EC measurements can be integrated into an AMS, allowing for real-time monitoring of udder health. This integration facilitates early intervention and targeted treatment, thereby improving herd management and milk quality. While EC testing is not a standalone solution, its combination with other diagnostic methods (such as SCC and CMT) enhances its effectiveness in mastitis detection.
Identifying the pathogens present
Identifying the pathogens present is essential for practitioners to make appropriate recommendations in terms of treatment and prevention.
Where possible, vets and farmers should work together, such that clear on-farm protocols are in place regarding aseptic sample collection in terms of both the “when” and the “how”. The how should be informed in part by the different grading of clinical mastitis recommendations (Table 1). However, other reasons to collect samples for testing exist. It is paramount that no contamination of samples is allowed to happen – from hands, equipment, other teats or environmental detritus.
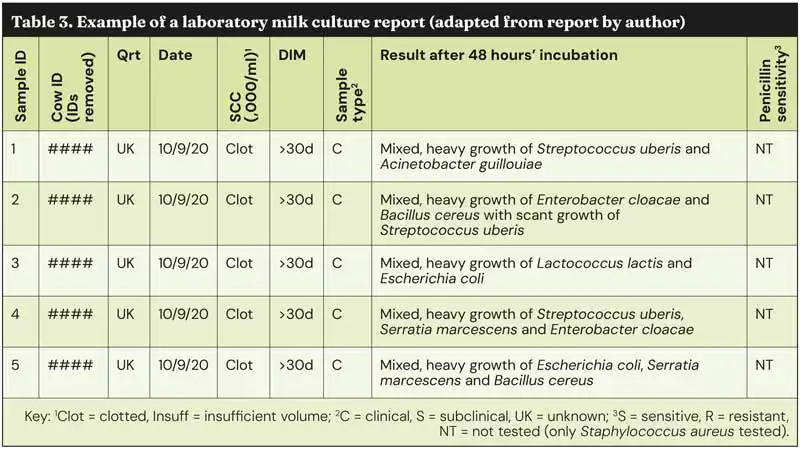
Typically, these samples would then be sent to the lab for incubation (at 37°C) for 18 to 24 hours, although for some species this would be increased to 48 hours. Samples would then be plated for bacteriological culture and sensitivity. Gram staining or PCR may also be carried out on the colonies grown. Testing with a certified diagnostic laboratory will provide the most accurate and reliable results, identifying all species present – and potentially providing sensitivity results (Table 3) – but this takes time.
Samples can also be collected and stored in a domestic freezer to create a “bank” of samples to be sent, as required, for understanding the herd-level picture or assessing a treatment failure.
New developments and technology have allowed for innovation and new approaches to mastitis testing on-farm using point-of-care tests. These new tests vary in price and function.
One such development provides an on-farm mechanism to culture and easily identify if the causative pathogens are Gram-positive or Gram-negative, informing treatment protocols in non-severe cases (Malcata et al, 2021).
The marketing company advises that accurate results are provided within 8 to 24 hours (8 to 12 for Gram-negative, and 24 for Gram-positive); they also report sensitivity of 82.0% and specificity of 76.8% for Gram-positive pathogens, with 83.3% and 94.3%, respectively, for Gram-negative pathogens (Vetoquinol, 2022).
Another rapid on-farm test reports the return of results within eight hours, identifying Gram-positive organisms within the usual space between milkings; as such, implying that the test would not delay treatment – which is usually administered via the intramammary route in the parlour – and reducing antibiotic use in cases that may well not require it.
The marketing company reports that this reduces economic losses by reducing days out of the tank, medicines spend and by reducing the time involved in managing the case (Lago and Godden, 2018; Zoetis Animal Health, 2025).
Artificial intelligence – more specifically machine learning – has been demonstrated to aid in the analysis of farm data, diagnosing the period of origin (dry period or lactational) and contagious or environmental pathogen types. Hyde et al (2020) reported 98% accuracy with a positive predictive value of 86% and negative predictive value of 99%, indicating a useful tool to aid practitioners, farmers and consultants alike.
Lastly, one new technological invention allows for rapid on-farm identification of bacterial pathogen type, antibiotic sensitivity, and can provide a vet-recommended protocol. It is important to note that this product still involves collaboration with the vet and the protocol is only provided if the vet has enabled the feature. This product returns results within 24 hours, using colour-change phenomena to analyse the sample and then send these results to the cloud, where the company’s algorithm analyses them to then report back results. The machine can be used to analyse both clinical cases and high SCC samples.
The manufacturer claims that use of the machine can reduce antibiotic use on-farm by 24%, with a significant reduction in BSCC (Bates et al, 2020; Mastatest, 2022).
Whether farmers and their vets opt for on-farm or laboratory-based testing of milk samples, it remains an essential part of mastitis diagnosis, prevention and control. One of the major benefits of testing samples from affected cows is the ability to be more targeted in our treatment approaches and to use selective treatment (ST) of clinical cases.
ST of clinical cases bases itself on the widely accepted evidence-based approach that many Gram-negative bacterial infections will self-cure without intramammary or systemic antibiotic use. NSAID use is still indicated, and ketoprofen, with its nil withhold, is often an appropriate choice.
The new on-farm tests, as mentioned previously, allow for rapid differentiation between Gram-positive and Gram-negative infections, and can allow prompt and appropriate interventions. Gram-positive infections should still be treated with narrow spectrum, category D antibiotics first line.
Several pros exist for the use of ST for clinical mastitis, including reduced waste milk due to withdrawal periods, improved responsible use of antimicrobials and improved antimicrobial stewardship. Nonetheless, some potential disadvantages also exist. Full debate of the disadvantages is beyond the scope of this article, but is covered extensively in the wider literature (de Jong et al, 2023; Roberts, 2025b).
Quantifying where and when infection is occurring
After mastitis has been diagnosed, and the causative pathogens identified, it is important to understand where and when the majority of infections occur – this will be essential to provide appropriate control and prevention advice, and is rooted in appropriate data collection and recording. Most farms will be milk recording with BSCC and ICSCC data available to input into software such as Interherd+ or TotalVet. However, clinical cases may be recorded elsewhere – and may not be inputted into on-farm software by the farmer – and this may require a deep dive into on-farm paper records, and/or the medicines records, to identify the true number of clinical cases and chronic or recurring cases.
Four main categories are used to identify where and when infection occurs:
- Dry period origin – presents as high numbers of cows that were dried off with a low SCC having a high SCC in the first 30 days post-calving.
- Lactation period origin – presents as new infections within the lactation period.
- Environmental – associated with dry period origin and lactation period – the dry cow environment or the milking cow environment.
- Contagious – primarily associated with lactation period origin and, specifically, the milking routine, although contagious pathogens can be associated with dry period origin.
Prevention
Only by understanding what is causing the infection, and when infection is occurring, can appropriate control and prevention measures be implemented.
In addition to the methods mentioned in this article, on-farm assessment and analysis is fundamental in working with clients to reduce the occurrence of clinical and subclinical mastitis on farms. Programmes such as the Mastitis Control Plan (MCP) provide a thorough and logical step by step approach to the on-farm visit and assessment, as well as advice for parlour visits. The MCP also provides a measure of quality and confidence to farmers that the MCP plan deliverer visiting their farm to work with them has undertaken further training in the area (DairyCo Mastitis Control Plan – Welcome, no date).
Farms involved in the MCP when it first launched reported a 22% reduction in cows affected by clinical cases in the first year of acting on the recommendations compared with control herds (AHDB, 2013).
Whether the MCP or other approaches are used, farm visits should appraise the data, results of milk testing, as well as observations gained on-farm and from discussion with the client, to provide recommendations. These should be prioritised and broken down into manageable and realistic steps, rather than overwhelming clients with chapter and verse advice rather than specific actions, metrics, and ways to measure and monitor the impact of change.
Conclusions
Mastitis remains one of the most economically impactful diseases affecting dairy farms globally. Furthermore, it is a significant contributor to the total antibiotic use on farm.
New developments in on-farm technology allow for rapid initial assessment of the causative pathogens which, in turn, can allow for selective treatment of clinical mastitis, reducing the use of antimicrobials on farm and encouraging responsible use.
Whether vets and farmers use traditional or new methods for the diagnosis and detection of clinical or subclinical mastitis on farm, a strong vet-farmer relationship is required for a mastitis investigation to understand where infection is occurring and to provide a realistic, actionable plan to tackle mastitis on farm.
Appeared on Vet Times (2025), Volume 55, Issue 24, Pages 6-12
References
- AHDB (2013). Mastitis control plan, tinyurl.com/3bt2svs9
- AHDB (2025). UK farmgate milk prices, tinyurl.com/2jf27n4k
- Bates A et al (2020). Selective and deferred treatment of clinical mastitis in seven New Zealand dairy herds, Prev Vet Med 176: 104915.
- Breen J (2014). Reducing mastitis in cows during post-calving period, Vet Times, tinyurl.com/46x5mnzp
- Ceva Animal Health (2018). Care of dairy cows at dry off, tinyurl.com/pvm63fu2
- DairyCo Mastitis Control Plan (no date). Welcome, tinyurl.com/wywyz5me
- Fernando RS et al (1982). Electrical conductivity of milk for detection of mastitis, J Dairy Sci 65(4): 659-664.
- Gard R (2023). What’s new in mastitis – the 34th British Mastitis Conference, Veterinary Practice, tinyurl.com/t554sybt
- GB Cattle Health and Welfare Group (2020). Fifth Report, tinyurl.com/5dmrxxhz
- Hyde RM et al (2020). Automated prediction of mastitis infection patterns in dairy herds using machine learning, Sci Rep 10(1): 4,289.
- Isaksson A et al (2021). The electrical conductivity of bovine milk in mastitis diagnosis, Acta Vet Scand 28(3-4): 455-457.
- De Jong E et al (2023). Invited review: selective treatment of clinical mastitis in dairy cattle, J Dairy Sci 106(6): 3,761-3,778.
- Lago A and Godden SM (2018). Use of rapid culture systems to guide clinical mastitis treatment decisions, Vet Clin North Am Food Anim Pract 34(3): 389-412.
- LLM Farm Vets (2021) Counting the cost of mastitis, tinyurl.com/4w6c2jey
- Malcata FB et al (2021). Laboratory-based evaluation of a simplified point-of-care test intended to support treatment decisions in non-severe bovine clinical mastitis, J Dairy Res 88(2): 170-175.
- Mastatest (2022). Mastatest launches in UK, tinyurl.com/2cnxc5em
- National Mastitis Council (2001). Guidelines on normal and abnormal raw milk based on somatic cell counts and signs of clinical mastitis, tinyurl.com/csyhnt6u
- Roberts J (2025a). The California mastitis test: what is the value?’, UK-VET Livestock 29(5): 184-193.
- Roberts J (2025b). Selective treatment of clinical mastitis in dairy cows, UK-VET Livestock 30(Suppl 2): S12-S20.
- Shook G and Seaman A (1983). The new DHI linear score for somatic cell count, J Dairy Sci 39(12): 22-23.
- Vetoquinol (2022). VetoSlide, tinyurl.com/yc43c4bb
- Zoetis Animal Health (2025) See it in action – Mastigram+, tinyurl.com/53jvc64w
