7 Oct 2019
Measurement of passive transfer of immunity in calves
Nicola Gladden reviews the measurement methods available to practitioners and discusses routine herd-based monitoring.

Image: Nisha / Adobe Stock

Calves are born almost agammaglobulinaemic, and ensuring adequate passive transfer of immunoglobulins within the first few hours of life is an important part of newborn calf management. Failure of passive transfer is associated with increased morbidity and mortality of calves, as well as having a negative impact on productivity.
Monitoring passive transfer at herd level is a useful tool to monitor the colostrum management and care of newborn calves on farm, and can highlight potential problems before increased calf mortality is observed. A number of methods are available for measuring passive transfer in calves and are discussed in this article.
Passive transfer of immunity (commonly termed “passive transfer”) refers to the process by which immunoglobulins are absorbed from the gastrointestinal tract of newborn calves.
Calves are born almost agammaglobulinaemic due to the inability of immunoglobulins to be transferred across the placenta in domestic ruminants, meaning passive transfer from the neonatal gut is essential.
The success of passive transfer depends on calves ingesting an adequate volume of good-quality colostrum before the ability to absorb macromolecules from the gut ceases. This has usually occurred by the time the calf is 24 hours old; however, optimum absorption occurs before 4 hours of age, so it is recommended farmers ensure adequate colostrum intake in the first 4 hours of life.
Reported prevalence of failure of passive transfer (FPT) in calves ranges, but, in some studies, is as high as 35%1-6. FPT is associated with increased morbidity and mortality5, and has a negative impact on productivity, as well as health and welfare; it has been estimated FPT results in economic losses of between €60 and €80 (between £53 and £71) per calf7.
Although FPT is often considered a problem concerning dairy herds, beef calves can also be affected8 – so passive transfer is an important consideration when investigating neonatal calf disease in a beef herd.
While assessment of passive transfer is important when investigating disease in neonatal calves, regular analysis is also a useful tool for monitoring farm colostrum management and can highlight potential problems before increased calf morbidity is seen.
A number of methods are available for monitoring passive transfer, and all have their advantages and disadvantages.
Radial immunodiffusion (RID) directly measures immunoglobulin concentration and is considered the gold standard testing method; using this method, an IgG concentration of 10g/L or greater is considered to be indicative of adequate passive transfer. However, RID is expensive and time-consuming, and, therefore, not routinely used commercially9.
A number of methods that indirectly measure immunoglobulin concentration – and are less expensive and easier to use – have been developed. This article reviews those most commonly available to practitioners in the UK.
A summary of test methods has been provided in Table 1.
| Table 2. Summary of testing methods used for passive transfer of immunity in calves | ||||
|---|---|---|---|---|
| Test | Sample type | Failure of passive transfer threshold | Advantages | Disadvantages |
| Zinc sulphate turbidity (ZST) test | Serum only | Less than 20 ZST units | Quick, easy, low cost. | Poor sensitivity. |
| Total protein concentration (standard handheld refractometer) | Serum | Less than 5.2g/dL | Quick, easy, low cost, can be done in-house, ideal for routine monitoring healthy calves. May be affected by dehydration or presence of inflammatory proteins in sick calves, although changing the test end point can improve sensitivity and specificity. | |
| Plasma | Less than 5.6g/dL | |||
| Total protein concentration (Brix refractometer) | Serum or plasma | Less than 8.5 per cent | Quick, easy, low cost, can be done in-house, refractometer can also be used to measure colostrum Ig concentration. | Not as well studied as other methods for measurement of total protein concentration in calves; therefore, less evidence for benefit over other test methods. |
| Gamma-glutamyl transferase | Serum or plasma | Less than 50U/L | Quick, easy, low cost, less affected by dehydration. | Requires specialist equipment (analyser); literature reports inconsistent reliability for indicating failure of passive transfer, confers no advantage over other methods. |
| Radial immunodiffusion | Serum | Less than 10g/L IgG | Gold standard. | Expensive, time-consuming and not readily available. |
Measurement of total protein concentration
In neonatal ruminants, absorption of immunoglobulin following colostrum ingestion results in an increase in globulin concentration. Analysis of total serum or plasma protein concentration indirectly measures this increase.
Measurement of total protein concentration – using either a traditional or Brix refractometer – can quickly and easily be done in the practice laboratory using either plasma or serum samples, and requires little specialist equipment.
Traditional handheld refractometers measure the refractive index of the sample as light passes through it. A shadow forms and the reading is taken where the shadow crosses the scale that can be seen through the refractometer eyepiece (Figure 1).

Most refractometers used in veterinary practice measure total protein concentration in units of grams per decilitre (g/dL). Both methods can be performed on farm if samples are stood upright for a few minutes to allow the serum to separate out and the blood to clot.
Studies have shown total protein measurement compares well to other methods of IgG assessment9,12,13; sensitivity and specificity are affected by the chosen end point.
Tyler et al (1996)12 compared test performance using different total serum protein concentrations. Using a test threshold of 5g/dL results in a test with high specificity (96%), but low sensitivity (59%) – meaning that, although few animals with FPT would be misclassified as having adequate passive transfer, a high proportion of calves with adequate passive transfer would be misclassified as having FPT.
Increasing the test threshold to 5.5g/dL has the opposite effect and results in a test with high sensitivity (94%), but reduced specificity (76%).
The optimal threshold for determination of FPT has been suggested to be 5.2g/dL11,12,14,15; however, it has been suggested if a plasma sample is used (as opposed to serum), the threshold used to indicate FPT should be increased to 5.6g/dL16.
Although total protein measurement is often thought to be of less value in sick calves, it has been shown to compare well with other test methods in this context17.
Due to the concentrating effect of dehydration, using a higher threshold of 5.5g/dL to indicate FPT has been shown to improve specificity and sensitivity in calves experiencing illness17.
A Brix refractometer measures sucrose concentration as a percentage concentration by mass. In samples such as serum or plasma that do not contain sucrose, the Brix refractometer approximates total solids as a percentage concentration.
An additional advantage of Brix refractometry is that the same equipment can be used for both colostrum quality and passive transfer.
Measurement of total serum or plasma protein concentration is quick, cheap and easy to perform using equipment found in most practice laboratories.
Reliability of identifying calves with FPT is comparable to other test methods available and this test performs particularly well in healthy calves. Due to its low cost and reliability, this is the testing method of choice for routine calf health monitoring9.
Zinc sulphate turbidity test
The zinc sulphate turbidity (ZST) test is offered by a number of UK laboratories and commonly used in practice to assess passive transfer in calves up to a week old. It was first described as a method for measuring passive transfer in calves nearly 50 years ago22; more recently, optimisation of the technique has been suggested23.
The ZST test provides an indirect measure of immunoglobulin concentration by measuring the turbidity reaction that occurs when bovine serum is added to a zinc sulphate solution.
The traditional test method uses colourimetry to compare the test sample with a control; although a more simplified, albeit more subjective, method has been suggested whereby passive transfer is considered to be adequate if newsprint cannot be read through a test tube in which calf serum and zinc sulphate are mixed12.
A number of factors can affect the ZST test – including the wavelength of light used to measure the turbidity test, concentration of the zinc sulphate solution, time allowed for the reaction to take place and sample haemolysis10,22.
Traditionally, samples with a test result less than 20 ZST units have been considered to be indicative of FPT23; however, although the sensitivity of the test is good, the specificity of the test when this threshold is used has been reported to be low9,12 – meaning a high number of calves with adequate passive transfer may be misclassified as having FPT.
In calves that are clinically sick, specificity of the ZST test has been shown to be reduced further, although sensitivity remains higher than 90%17. Reducing the test threshold has been shown to increase the specificity of the ZST test9,23 and a threshold of 12.5 units has been suggested to be optimal23.
Increasing the zinc sulphate concentration used in the testing method has also been demonstrated to improve test specificity10,23.
In practice, the ZST test is relatively quick and cheap to perform, and requires no specialist equipment beyond that required to obtain a blood sample. It is, therefore, useful in situations where referral to an external laboratory is available, but in-house analysis of samples may not be possible.
Gamma-glutamyltransferase
Colostrum contains a high concentration of gamma-glutamyltransferase GGT24 – and serum GGT concentration in neonatal calves increases rapidly following colostrum ingestion.
Disagreement exists in the literature with regards to the value of GGT concentration as a measure of FPT in calves. Parish et al25 found good correlation between serum GGT and immunoglobulin concentrations, and defined reference concentrations of GGT indicative of FPT for different age groups of calves (Table 2).
| Table 2. Suggested reference serum gamma-glutamyltransferase (GGT) concentration in neonatal calves25 | |
|---|---|
| Age | Reference serum GGT reference concentration |
| 24 hours | Greater than 200U/L |
| 4 days | Greater than 100U/L |
| 7 days | Greater than 75U/L |
| Failure of passive transfer (any age) | Less than 50U/L |
An Irish study also found, for calves younger than five days of age, serum GGT concentration was a good indicator of FPT9.
Other studies, however, have found serum GGT concentration in neonatal calves correlates poorly with serum immunoglobulin concentration13,26. Serum GGT concentration is, however, less affected by dehydration or illness than other test methods and may be of use in sick calves.
Although serum GGT concentration is highest in the first 24 hours of life and declines thereafter, it can take up to five weeks for serum GGT concentration to become similar to those of adult cattle27, which needs to be considered when interpreting biochemistry results from young calves.
Serum GGT concentration can be measured using in-house analysers, if available, or referred to an external laboratory. Measurement is relatively inexpensive and multiple samples can be analysed at the same time in some analysers, improving the speed and convenience of the test. However, little evidence exists to recommend the use of measurement of serum GGT concentration in preference to other methods for the purposes of assessment of passive transfer.
Other methods
Serum globulin concentration has been demonstrated to have a similar sensitivity and specificity as other, more commonly used testing methods9.
Similar to GGT, serum globulin concentration can be analysed by an in-house analyser or external laboratory and is relatively inexpensive, but does not offer any advantages over and above other testing methods available.
Routine herd-based monitoring of passive transfer of immunity
Regular monitoring of passive transfer is associated with a lower within-herd prevalence of FPT1 and can be used to monitor the efficacy of farm colostrum management.
Rather than focusing on individuals, monitoring of passive transfer on a herd basis aims to identify the proportion of calves in the herd that do not meet the test threshold indicative of adequate passive transfer.
A target level of 80% to 90% of calves having adequate passive transfer has been suggested28,29; the author’s practice sets a target of more than 85% of calves having adequate passive transfer.
If the proportion of calves with adequate passive transfer falls below the determined target, further investigation is warranted.
Adequate numbers of calves need to be sampled to be representative of the herd; sampling in groups of 12 has been recommended28. Sporadic sampling of individuals is to be avoided; if the herd is small, samples should be accumulated until 12 are obtained.
Either serum can be frozen and stored, or samples can be tested at the time they are obtained and results accumulated before interpretation. How frequently samples are taken is dependent on the size of the herd and the number of calves born, as well as the practicalities of attendance to the farm; in large year-round calving herds, however, sampling every six to eight weeks can be useful to enable a temporal trend to be obtained (Figures 2a and 2b).
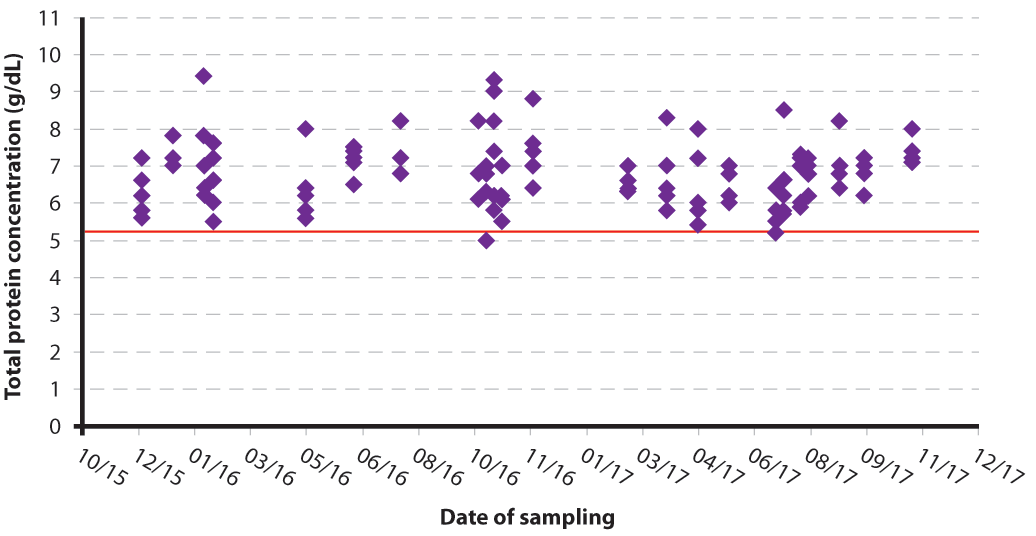
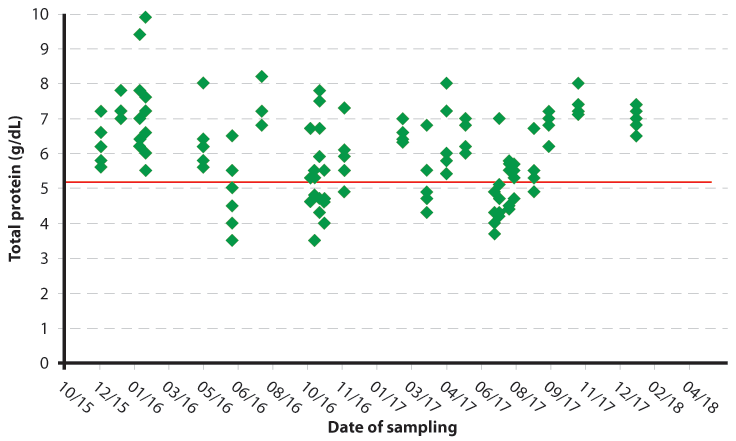
Measurement of total protein concentration is the preferred test method for routine herd-based monitoring of passive transfer. Both centrifuged and non-centrifuged samples have been shown to produce similar results30; therefore, samples can either be analysed at the practice or can be left to clot and tested on farm without the need for a centrifuge.
Clinically normal calves aged between 24 hours and 7 days old should be sampled for routine monitoring.
Assessment of passive transfer using total protein concentration is a cheap and straightforward method to assess calf health at herd level. Herd level passive transfer monitoring can be used to stimulate discussion about calf health and can be a good entry point for the practitioner in developing a calf component to the farm’s herd health planning.
- This article appeared in Vet Times (2019), Volume 49, Issue 40, Pages 14-15.
References
- Beam AL, Lombard JE, Kopral CA et al (2009). Prevalence of failure of passive transfer of immunity in newborn heifer calves and associated management practices on US dairy operations, J Dairy Sci 92(8): 3,973-3,980.
- Waldner CL and Rosengren LB (2009). Factors associated with serum immunoglobulin levels in beef calves from Alberta and Saskatchewan and association between passive transfer and health outcomes, Can Vet J 50(3): 275-281.
- Lawrence K, Broerse N, Hine L et al (2017). Prevalence of failure of passive transfer of maternal antibodies in dairy calves in the Manawatu region of New Zealand, NZ Vet J 65(1): 1-5.
- Cuttance EL, Mason WA, Laven RA et al (2017). Prevalence and calf-level risk factors for failure of passive transfer in dairy calves in New Zealand, NZ Vet J 65(6): 297-304.
- Brignole TJ and Stott GH (1980). Effect of suckling followed by bottle feeding colostrum on immunoglobulin absorption and calf survival, J Dairy Sci 63(3):451-456.
- MacFarlane JA, Grove-White DH, Royal MD and Smith RF (2015). Identification and quantification of factors affecting neonatal immunological transfer in dairy calves in the UK, Vet Rec 176(24): 625.
- Raboisson D, Trillat P and Cahuzac C (2016). Failure of passive immune transfer in calves: a meta-analysis on the consequences and assessment of the economic impact, PLOS One 11(3): e0150452.
- Corbishley A, Tomlinson MS, Forrest J et al (2017). Failure of passive transfer: not just a dairy problem, Cattle Pract 25(3): 141-145.
- Hogan I, Doherty M, Fagan J et al (2015). Comparison of rapid laboratory tests for failure of passive transfer in the bovine, Ir Vet J 68(1): 18.
- Weaver DM, Tyler JW, VanMetre DC et al (2000). Passive transfer of colostral immunoglobulins in calves, J Vet Intern Med 14(6): 569-577.
- Hernandez D, Nydam DV, Godden SM et al (2016). Brix refractometry in serum as a measure of failure of passive transfer compared to measured immunoglobulin G and total protein by refractometry in serum from dairy calves, Vet J 211: 82-87.
- Tyler JW, Hancock DD, Parish SM et al (1996). Evaluation of 3 assays for failure of passive transfer in calves, J Vet Intern Med 10(5): 304-307.
- Cuttance EL, Mason WA, Denholm KS and Laven RA (2017). Comparison of diagnostic tests for determining the prevalence of failure of passive transfer in New Zealand dairy calves, NZ Vet J 65(1): 6-13.
- Calloway CD, Tyler JW, Tessman RK et al (2002). Comparison of refractometers and test endpoints in the measurement of serum protein concentration to assess passive transfer status in calves, J Am Vet Med Assoc 221(11): 1,605-1,608.
- Buczinski S, Gicquel E, Fecteau G et al (2018). Systematic review and meta-analysis of diagnostic accuracy of serum refractometry and Brix refractometry for the diagnosis of inadequate transfer of passive immunity in calves, J Vet Intern Med 32(1): 474-483.
- MacFarlane JA, Grove-White DH, Royal MD and Smith RF (2014). Use of plasma samples to assess passive transfer in calves using refractometry: comparison with serum and clinical cut-off point, Vet Rec 174(12): 303.
- Tyler JW, Parish SM, Besser TE et al (1999). Detection of low serum immunoglobulin concentrations in clinically ill calves, J Vet Intern Med 13(1): 40-43.
- Denholm KS, Hunnam JC, Cuttance EL and McDougall S (2017). Influence of preservation methods on the quality of colostrum sourced from New Zealand dairy farms, NZ Vet J 65(5): 264-269.
- Bielmann V, Gillan J, Perkins NR et al (2010). An evaluation of Brix refractometry instruments for measurement of colostrum quality in dairy cattle, J Dairy Sci 93(8): 3,713-3,721.
- Vandeputte S, Detilleux J and Rollin F (2014). Investigation of colostrum quality in beef cattle by radial immunodiffusion and Brix refractometry, Vet Rec 175(14): 353.
- Deelen SM, Ollivett TL, Haines DM and Leslie KE (2014). Evaluation of a Brix refractometer to estimate serum immunoglobulin G concentration in neonatal dairy calves, J Dairy Sci 97(6): 3,838-3,844.
- McEwan AD, Fisher EW, Selman IE and Penhale WJ (1970). A turbidity test for the estimation of immune globulin levels in neonatal calf serum, Clin Chim Acta 27(1): 155-163.
- Hogan I, Doherty M, Fagan J et al (2016). Optimisation of the zinc sulphate turbidity test for the determination of immune status, Vet Rec 178(7): 169.
- Blum J and Hammon H (2000). Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine and metabolic parameters in neonatal calves, Livest Prod Sci 66(2): 151-159.
- Parish SM, Tyler JW, Besser TE et al (1997). Prediction of serum IgG1 concentration in Holstein calves using serum gamma glutamyltransferase activity, J Vet Intern Med 11(6): 344-347.
- Vandeputte S, Detilleux J and Rollin F (2011). Comparison of four refractometers for the investigation of the passive transfer in beef calves, J Vet Intern Med 25(6): 1,465-1,469.
- Thompson JC and Pauli JV (1981). Colostral transfer of gamma glutamyl transpeptidase in calves, NZ Vet J 29(12): 223-226.
- McGuirk SM and Collins M (2004). Managing the production, storage, and delivery of colostrum, Vet Clin North Am Food Anim Pract 20(3): 593-603.
- Godden S (2008). Colostrum management for dairy calves, Vet Clin North Am Food Anim Pract 24(1): 19-39.
- Wallace MM, Jarvie BD, Perkins NR and Leslie KE (2006). A comparison of serum harvesting methods and type of refractometer for determining total solids to estimate failure of passive transfer in calves, Can Vet J 47(6): 573-575.
Meet the authors
Nicola Gladden
Job Title