27 Aug 2018
Jacqueline Matthews suggests diagnostic techniques for the improved treatment and management of helminths affecting on-farm cattle.

Image: Rebecca Matthews.
Parasitic helminths are an important cause of production loss in livestock.
A range of worm species can infect cattle. Often, the impact of infection is subclinical, with negative effects on growth and milk yield. In heavy infections, clinical signs can develop. In intensive farming systems, control measures must be implemented to lower worm infection levels to reduce the impact of infection.
For several decades, control has been dependent on the regular application of broad-spectrum anthelmintics; however, frequent treatments with these medicines have led to drug resistance developing.
Reports have indicated anthelmintic resistance in gastrointestinal nematodes is increasing worldwide. Although resistance is less recognised in cattle than in sheep worms, surveys have identified drug resistance – especially in the small intestinal parasite Cooperia oncophora. For example, an EU-wide study reported low efficacy of ivermectin and moxidectin in more than 50% of farms tested in Germany, France and the UK. Cooperia species were the most frequently identified worms after treatment, with the more pathogenic worm Ostertagia ostertagi also detected on some UK and German farms.
In addition to these worrying trends in nematodes, Fasciola hepatica resistance to the most potent flukicide, triclabendazole, is common in sheep populations in some parts of the UK. While treatment failure has been mostly reported in sheep, as both host species are infected with the same fluke populations when co-grazed, resistance is likely in cattle, too. No new novel anthelmintic compounds are near commercialisation for cattle, so once efficacy of effective products is lost, significant losses could incur as worm infection levels rise.
To reduce selection pressure for resistance, evidence-based approaches to worm control need to be taken. This requires a balance to be struck between applying treatments to reduce infection levels and minimise production loss, with the need to preserve anthelmintic efficacy. Because of local differences in helminth prevalence and management, control plans need to be developed on a farm-by-farm basis, involving direct input from a vet or qualified animal health advisor. To develop effective plans, knowledge of epidemiology and individual farm history is required, supported by diagnostic tests to help build a picture of:
Control programmes should contain a blend of diagnostic-led targeted treatments, combined with strategic all-group treatments in some stock at certain times of year. Programmes must include grazing management strategies that aim to break worm life cycles to reduce infection in the environment. This article reviews diagnostics in helminth control programmes on UK cattle farms.
In the UK, the commonest helminths encountered are the gastrointestinal nematodes, O ostertagi and C oncophora – the main cause of parasitic gastroenteritis.
The abomasal worm O ostertagi is the most pathogenic, and control programmes need to ensure this species does not build up in significant burdens in youngstock. C oncophora is less pathogenic, but some studies have shown this worm can cause weight loss when burdens are high.
Other less prevalent nematode species can be present along with these; co-infection increases production losses incurred. In some regions, the bovine lungworm Dictyocaulus viviparus is an important pathogen of calves and worm-naive adult cattle. This can cause severe respiratory distress and significant production loss. Lungworm is more common in wetter western regions and outbreaks can be unpredictable.
Until exposed to natural infection or vaccination, all cattle are at risk of disease. D viviparus should be suspected with coughing or respiratory signs in cattle at grass, especially first season grazing calves. It can cause disease and losses in adult cattle, with the cost of an outbreak being estimated at €111 (£99) per cow (Bovilis, 2018). Once exposed, cattle develop immunity, but can subsequently develop disease if immunity wanes – especially when pasture infectivity is high. Farms with a history of lungworm should consider vaccination as an integral part of their control programme.
Fasciola hepatica (liver fluke) is an important cause of production loss in UK cattle, causing reduced milk yield, poor growth and lowered fertility. In some regions, the rumen fluke Calicophoron daubneyi is present. This species does not generally cause clinical signs. When disease has been reported, it has been associated with high burdens of immature larvae causing diarrhoea and ill thrift in young stock. Liver and rumen fluke are often found together and, because their eggs are similar, misdiagnoses can occur.
It is important to discriminate the species using diagnostic tests as only oxyclozanide can be used to treat rumen fluke. Both flukes have indirect life cycles, involving amplification in an aquatic snail host, so their geographical distribution is associated with proximity to water bodies and wetlands. Liver fluke is by far the more important clinical threat to cattle.
Several types of test can be used to detect worm infections, some of which are useful in investigating anthelmintic effectiveness. These are summarised in Table 1.
| Table 1. Tests commonly used for detecting helminth infections in cattle | |
|---|---|
| Diagnostic test | Headlines |
| Faecal egg count test | • Different methods for nematodes and flukes (flotation versus sedimentation techniques). • Good test for distinguishing different types of fluke eggs. • Test does not detect prepatent infections. • Test can be used for efficacy testing. |
| Plasma pepsinogen analysis | • Test used for detecting ostertagiosis in young cattle. • Not specific. • Test of no value in adult cattle. |
| Baermann test | • Test for detecting lungworm larvae in faecal samples. • Sensitivity can be low, but using a large volume (more than 30g) of well-mixed faeces improves sensitivity. • Test does not detect prepatent infection. |
| Coproantigen test | • Test detects liver fluke antigens in faeces. • Test can be used for efficacy testing. |
| Antibody analysis | • Various tests available for detecting worm-specific antibodies in serum or milk. • Test does not inform on level of infection. • Test is good for population-based analysis. • Test is of no value in efficacy testing. |
Diagnostic tests need to be sensitive, specific and repeatable. Reflect on these each time a test is considered.
Patent helminth infections can be detected by examining faecal samples for worm egg presence. Egg count tests span flotation methods to detect nematode eggs and sedimentation methods to detect fluke eggs. Faecal egg count (FEC) tests are very useful for measuring egg shedding to provide information for:
FEC tests have no value in detecting pre-patent infection, so cannot be used to detect early disease. Worm egg shedding levels do not correlate well with total burden – they only provide an indication of the presence of egg-producing adult females, nor do they correlate linearly with liveweight gain in young cattle. Most nematode species cannot be identified on egg morphology.
For identification, eggs need to be cultured to larvae for classification; however, in practice, it is unlikely species identification is required, unless undertaking a detailed assessment of anthelmintic efficacy. In contrast, liver fluke eggs can be differentiated from rumen fluke eggs on the basis of colour; rumen fluke eggs are identified as a paler egg compared to the golden colour of liver fluke eggs (Figure 1).
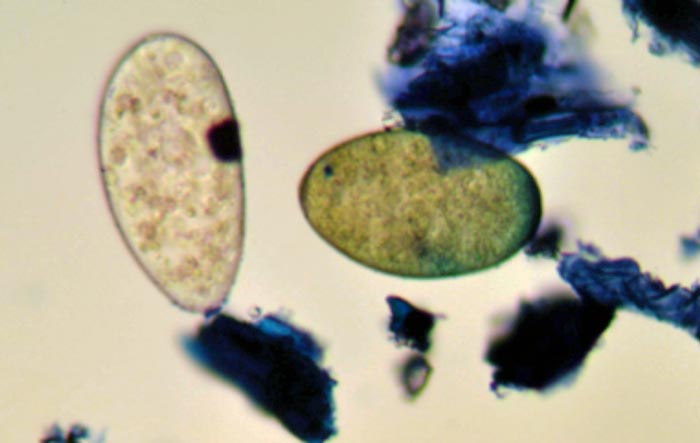
FEC tests indicate the approximate concentration of eggs in dung at sampling. To assess total daily contamination, egg per gram (EPG) results can be multiplied by the fresh weight of faeces produced each day. Cattle excrete approximately 5% liveweight in faeces each day, so a 200kg calf with an FEC of 200 EPG would be estimated to shed 2,000,000 worm eggs in one day.
For nematodes, FEC tests can be used late spring through summer in first season grazing calves to identify contamination levels on to pasture to inform management decisions and treatment applications. The value of these tests to guide treatment decisions in milking cows has been indicated in studies, where different tests (FEC test versus plasma pepsinogen analysis versus detection of O ostertagi antibodies) were compared in cows postpartum (Mejía et al, 2011). When cows were partitioned based on worm egg shedding (EPG of 0 versus more than 0), a significant difference (approximately 800L milk/cow/lactation) was found.
Measurement of plasma pepsinogen and O ostertagi-specific antibodies did not provide informative data for targeting treatments. Nematode egg count analysis at housing is of no value because the developmental stages that need anthelmintic targeting in autumn to avoid type-2 ostertagiosis are larvae.
Helminth eggs are not evenly distributed in faeces, so always ensure samples taken for analysis are representative. This can be achieved by taking three to four sub-samples from across a dung pile, preferably 10g to 14g (a heaped dessert serving spoon’s worth) in total and mixing these in the transfer receptacle.
As eggs hatch quickly at warm temperatures, samples should be taken as fresh as possible (ideally, within an hour of excretion) and transferred to the laboratory within 48 hours. To reduce egg hatching (and under-estimation of FEC), samples should be transferred in containers with no air – a sealed plastic bag with all air excluded or a hard container filled to the top. If samples do not go immediately to the laboratory, they should be kept cool (40°C). At the laboratory, faeces should be mixed well prior to taking any sub-samples for weighing.
A sensitive method should be used to reduce inherent variability introduced by using multiplication factors to reach the estimated EPG value. A large multiplication factor (such as ×50 and ×100) is used in the commonest nematode egg counting method used in practice, the modified McMaster test. Several protocols are available to improve the sensitivity of flotation methods for counting nematode eggs, which include FLOTAC that has a minimum egg detection limit of 5 EPG or FECPAKG2, sensitive to 20 EPG. For older cattle, which often have low worm egg shedding, it is recommended a method with a minimum egg detection limit of 1 EPG to 10 EPG be used.
Fluke eggs can be counted using various sedimentation methods. These are useful in distinguishing liver fluke from rumen fluke, but are time-consuming and only detect patent infection. During the 10 to 12-week pre-patent period, false negatives occur. Liver fluke egg excretion is intermittent and eggs can be retained in the gall bladder, further increasing the potential for false negative results. Sensitivity of the standard sedimentation methods for F hepatica eggs varies from 30% to 70%, depending on the protocol, volume of faeces examined and level of infection in the population. By examining more than 30g faeces, test sensitivity can be increased to 90% (Rapsch et al, 2006).
An alternative to sedimentation egg count methods is the ”coproantigen” test, which measures F hepatica antigens present in faeces using antibodies. When first developed as a research tool (Mezo et al, 2004), this test was reported be 100% sensitive in detecting cattle infected with two or more F hepatica and was shown to be specific. An adaptation of this test has been developed commercially.
The performance of the commercial protocol was assessed by Mazeri et al (2016), who reported sensitivity estimates approximately 77% in different seasons, similar to results reported in Australia (Palmer et al, 2014). Using a lower cut off (manufacturer’s cut off ×0.67), Palmer et al (2014) demonstrated sensitivity could be increased to 87%. Both studies reported high specificity at approximately 99%. Results, so far, suggest the F hepatica coproantigen test does not cross-react with C daubneyi antigens.
In all cases, FEC data must be interpreted in the context of a risk assessment for the likely severity of contamination/infection alongside pasture history, animal age, production level and time of year.
For nematodes, the FEC reduction test (FECRT) is the method of choice for assessing anthelmintic efficacy. Studies have shown uptake on UK cattle farms is very low (Easton et al, 2018). More effort needs to be made to test anthelmintic sensitivity – especially where suspicion exists that treatments are not as effective as they once were.
As a ”look-see”, faecal samples can be taken one to two weeks post-treatment from approximately 10 animals that graze together. This provides a rough estimate of drug effectiveness. If positive FEC are identified after treatment, a FECRT should be done. Here, take individual samples from as many animals as possible (more than 15).
Ideally, each should have a FEC of more than 100 EPG at treatment. Administer anthelmintics at 100% to 110% the recommended dose, and sample dung at treatment and 10 to 17 days later, depending on the anthelmintic (10 days after benzimidazoles, 14 to 17 days after other classes).
The mean percentage FEC reduction obtained by comparing day 0 and day 14 to 17 counts is calculated and resistance reported if the per cent values fall below 95. Data interpretation is often affected by low worm egg shedding and high aggregation of FEC in cattle – that is, most animals have zero/low FEC and a minority (less than 20%) have higher counts, more than 200 EPG. To overcome this, a method with a minimum egg detection limit of 10 EPG should be used. This test does not enable definition of nematode species; faecal culture can done to generate larvae to provide information on which species survive treatment.
Because FECRTs are labour intensive and relatively expensive, they have poor uptake. To reduce cost, pooled samples can be counted. A US study looked at this for nematode eggs using 14 groups of cattle comparing individual and pooled sampling (George et al, 2017). Little difference occurred in mean FEC reported, with a 98% agreement between average FEC based on individual counts versus the pooled count.
More than 95% agreement exists in efficacy level reported between the methods. Use of pooling reduced the number of tests performed by 79%. Ideally, unless a calibrated tool is supplied to measure samples on site, faeces should be submitted individually and weighed out for pooling at a laboratory (Panel 1).
1. Collect samples fresh from a minimum of 15 animals, where possible.
2. The same animals must be sampled before and after treatment (for interval, see main text). If a sample is not obtained from an original animal after treatment, do not use the first faecal egg count (FEC) in calculating efficacy.
3. Collect and transfer individual samples at 40°C.
4. Mix each sample thoroughly (ideally with a homogeniser) for 15 seconds at the laboratory.
5. To pool samples, weigh 1g faeces from each animal and combine in one container.
6. Mix the pooled sample thoroughly (ideally with a homogeniser) for one minute.
7. Count the pooled sample using the selected method. Statistical analysis suggests a minimum of 140 eggs should be counted in the pre-treatment sample to provide statistical validity of the mean FEC. Therefore, repeated slides/chambers should be read in the pre-treatment count until a total of 140 eggs are counted, with all eggs counted on the final slide/chamber. Record the number of slides/chambers used and the same number of slides/chambers are counted in the post-treatment count. For example, count pre-treatment slide one and slide two until 140 eggs is reached (use total from final slide – for example, if 56, 56, 50 eggs counted over three slides/chambers, use 162 eggs as total then multiply total using multiple for method to estimate EPG). Count the same number of slides in the post-treatment analysis. Count total number of eggs on all slides and multiply using a multiple for the method to calculate EPG.
8. Calculate percentage FEC reduction as:
[(pre-treatment composite FEC (EPG) – post-treatment composite FEC (EPG))
+ pre-treatment composite FEC (EPG)] × 100
For liver fluke, the coproantigen test has been evaluated for efficacy testing and has proved promising, with one study demonstrating fluke antigen levels falling by seven days of triclabendazole treatment (Brockwell et al, 2013). This test is faster than a FECRT using standard sedimentation methods, which requires three weeks before the second sampling is done to take account of fluke eggs being released from dead adult fluke or the gall bladder.
A clinical history of coughing in cattle at grass from mid-summer onwards is highly indicative of D viviparus infection. Detection of first stage larvae (L1) in faecal samples using the Baermann technique can support diagnosis. L1 are present in fresh dung, and are 300μm to 450μm long and approximately 25μm wide. They contain dark granules in their intestinal cells. A minimum of 10g (ideally 30g) of well-mixed faeces is required for the test, which does not detect prepatent infection.
The test is not particularly sensitive, especially using lower volumes of dung, and negative L1 counts do not exclude infection when it is suspected clinically. Cases can present in the prepatent and post-patent phases, or may have been previously exposed (reinfection syndrome), and in these L1 will not be detected in faecal samples.
Diagnostics based on the detection of worm-specific antibodies in serum or milk are available for some helminths. These assays indicate exposure, do not provide an accurate measure of infection and can be associated with false positive results. They have no use in assessing efficacy.
Tests that measure specific antibody in serum and/or milk have been developed to detect exposure to O ostertagi, D viviparus and F hepatica. These are valuable in studying infection at the population level or in investigating prevalence. Bulk milk sample testing has been useful in research studies to inform on risk factors for F hepatica exposure in dairy cattle, and associated higher rainfall, wet grazing, presence of beef cattle, access to stream/ponds and smaller herd size with increased risk of exposure.
These tests are also helpful in establishing whether groups of cattle have been exposed to fluke or nematode infections over a grazing period and inform on future treatment – or, in the case of D viviparus, vaccination strategies. They can be used to give a long-term view on the effectiveness of control measures; for example, in previously-infected animals given a flukicide then moved to fluke-free pastures. A successful, specific antibody should take several months (three to six) to fall and this can be monitored by detecting anti-F hepatica antibodies in bulk milk samples.
The inspection of cattle livers in European abattoirs is mandatory. Liver inspection data can be used in practice to provide feedback on levels of infection on a farm and offers a useful tool in evaluating the effectiveness of control programmes.
A farm-specific plan should be drawn up each year with the aim of targeting anthelmintics when required based on parasite epidemiology, local factors (location, clinical history, season, weather and type/age of stock) and supported by the results of diagnostic tests.
For nematodes, first and second grazing season cattle are the focus for worm control. In the first season, the risk of developing disease depends on when calves were born and whether they co-graze with their dams. Highest risk groups are weaned calves on pasture grazed by cattle in the previous 12 months.
All-group treatments can be administered strategically (from three weeks after turn out) in the first half of the season (to reduce contamination); treatment frequency will depend on persistence of the anthelmintic formulation used. The application of effective anthelmintics at this stage reduces nematode egg shedding up to mid-summer, when most overwintered larvae will have died. Keep in mind that all-group treatments provide a strong selection pressure for drug resistance.
Alternatively, the decision to treat calves at grazing can be made on growth monitoring and FEC analysis. Liveweight gain is a good indicator of infection (where adequate nutrition is present and no other disease), so regular monitoring is key in assessing whether treatments are required. Weighing can be supported by FEC analysis to monitor contamination and effectiveness of control measures.
After grazing, first season grazing calves will require an all-group treatment to target O ostertagi larvae in the abomasum. Where pasture contamination risk has been perceived to be high, this treatment may also be needed in second season grazers. Macrocyclic lactones are the anthelmintic of choice for housing treatment as other classes do not have activity against all stages of O ostertagi larvae. Macrocyclic lactones will also have activity against common ectoparasites, which may pose a problem at housing.
Treatment with the macrocyclic lactone eprinomectin is popular on dairy farms due to its zero-day milk withdrawal period. However, blanket use of anthelmintics in milking cows is not recommended as not all animals have worm burdens requiring treatment; a study showed the benefit of treating milking cows is associated with individual infection level, which can be assessed by FEC testing (Verschave et al, 2014).
As cattle do not generally develop immunity to liver fluke, all ages of animals must be considered for treatment. Infective metacercariae reach peak pasture levels in autumn, so housing is an important time to consider treatment – especially following wet seasons, which promote snail survival and development.
Unlike macrocyclic lactones, flukicides have no persistent activity after treatment and animals can become reinfected quickly from contaminated pasture; therefore, anti-fluke treatments should be administered after housing. Apart from triclabendazole, flukicides are not highly effective against immature fluke. Because of resistance issues, triclabendazole use should be limited in cattle where chronic infections can be targeted, an exception being young stock on high-risk pasture or animals with acute infection in autumn.
Consequently, other flukicides should be applied six weeks after housing to allow flukes ingested at the end of the grazing season to develop to a stage where they will be sensitive to the dewormer. Most flukicides available in the UK are not licensed for milking cows, and milk withdrawal periods can be substantial. Information on dairy cattle flukicides is available from the Control of Worms Sustainably (2017).
Later in the housing period, liver fluke testing can help detect residual infections to guide the application of flukicides before turnout (hence reducing contamination of pasture the following season). A faecal sedimentation test here has the advantage of detecting rumen fluke and fluke eggs. Animals should only be treated for rumen fluke following a positive FEC test. Oxyclozanide should be applied on veterinary advice when a diagnosis has been made.
Mild winters facilitate survival of fluke-infected snails. This can lead to infections early in the grazing season. In these circumstances, animals can be tested for infection (FEC test, coproantigen test) approximately 12 weeks after turnout to inform on the need to apply treatment. On farms where heavy infection is already identified, an effective flukicide treatment two to three months after turnout can help reduce contamination and subsequent snail infection for the rest of the year.
As indicated, vaccination against lungworm should be undertaken on farms with a history of infection by this parasite. In other cases, Baermann analysis (in disease outbreaks) and antibody tests (to detect infection at the population level) can be used to inform whether vaccination is required.
First-year calves should be vaccinated each year. The parasite can be controlled using broad-spectrum anthelmintics, but this is risky in endemic areas because of the unpredictable nature of transmission and relatively few larvae can cause clinical signs.
Indeed, repeated treatments with effective persistent anthelmintics can limit exposure to lungworm antigens to such an extent that animals remain susceptible beyond the their first season. In these cases, vaccination prior to turnout in the second grazing season may be needed if parasite exposure is suspected or proven by testing.
In all cases, the risk of helminth disease can be reduced by avoiding contaminated grazing. Low-risk pastures – such as new-seeded fields, aftermath (not grazed since the previous year) and pastures not grazed by cattle for more than a year – should be prioritised for susceptible younger stock. Mixed/alternate grazing with sheep can reduce infectivity for nematode infections, but this may increase liver fluke risk. Keep in mind fields rested for a year containing snail habitats pose a risk for fasciolosis,and action should be taken to reduce environmental infection with snails by fencing off wet areas and/or improving drainage.
FEC tests can be used to support treatment decisions at quarantine, or faecal samples can be taken two to three weeks after a blanket quarantine treatment to assess if anthelmintics have been effective in reducing nematode egg shedding. In the case of F hepatica, quarantine actions should take a risk-based approach and treatment with non-triclabendazole products administered if fluke is suspected or detected by FEC, coproantigen or antibody testing in incoming cattle. As liver fluke eggs can be excreted up to three weeks after effective treatment, new cattle should be kept off pasture for four weeks (Table 2).
| Table 2. How diagnostic tests can be used in integrated worm control plans on cattle farms | |
|---|---|
| Test result or observation | Action |
| Weight loss (especially in first season calves) | Faecal egg count (FEC)? Treat for nematodes. |
| Coughing at grass | Baermann test and treat for lungworm. Implement vaccination programme? |
| Nematode FEC test + (first or second season calves and postpartum milking cows) | Establish eggs per gram (EPG) threshold for treatment (base on farm factors, such as grazing history and stocking density) and treat at group or individual level. |
| Liver fluke FEC or coproantigen test in autumn and spring + | Treat with flukicide (not triclabendazole). Implement snail control measures. |
| Rumen fluke FEC or coproantigen test in autumn and spring + | Treat with oxyclozanide. Implement snail control measures. |
| Serum antibody test for Ostertagia ostertagi + | Depending on time of year implement strategic treatments. Supplement with FEC tests to detect active infection? Treat where infection indicated and implement better grazing management. |
| Serum antibody tests Dictyocaulus viviparus + | Depending on time of year, implement vaccination or strategic treatments. May supplement with Baermann test to detect active infection. Treat where infection indicated and implement better grazing management. |
| Serum antibody tests liver fluke + | Depending on time of year, implement strategic treatments. May supplement with faecal testing to detect active infection. Treat where infection indicated. Implement better grazing management and snail control measures. |
| Slaughterhouse results + for liver fluke | Apply strategic treatments and/or routine diagnostic testing at housing and other times as required. Implement better grazing management and snail control measures. |
A worm control programme should be an integral part of all herd health plans. Diagnostics is increasingly important in assisting decision making. Tests can be exploited to garner evidence of infection and results interpreted in the context of grazing, clinical and treatment history. Ideally, plans should include tests to assess anthelmintic effectiveness.
Developing a reliable sustainable plan is not straightforward and the on-farm situation can change over time. Local knowledge can be supported by information obtained from online forecasting, such as the National Animal Disease Information Service (2018). This provides information and recommendations for treatment based on meteorological data.
Adoption of more evidence-based worm control protocols requires better communication between farmers and advisers, so knowledge on the values and limitations of the protocol is understood. Because of the potential complexity in these protocols compared a regularly treat all approach, a change in mindset needs to occur to enable uptake, which requires appropriate dialogue between the farmer and the advisor.